ImmunoPET: Concept, Design, and Applications
- PMID: 32202104
- PMCID: PMC7265988
- DOI: 10.1021/acs.chemrev.9b00738
ImmunoPET: Concept, Design, and Applications
Abstract
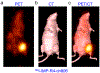
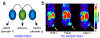
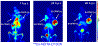
Immuno-positron emission tomography (immunoPET) is a paradigm-shifting molecular imaging modality combining the superior targeting specificity of monoclonal antibody (mAb) and the inherent sensitivity of PET technique. A variety of radionuclides and mAbs have been exploited to develop immunoPET probes, which has been driven by the development and optimization of radiochemistry and conjugation strategies. In addition, tumor-targeting vectors with a short circulation time (e.g., Nanobody) or with an enhanced binding affinity (e.g., bispecific antibody) are being used to design novel immunoPET probes. Accordingly, several immunoPET probes, such as 89Zr-Df-pertuzumab and 89Zr-atezolizumab, have been successfully translated for clinical use. By noninvasively and dynamically revealing the expression of heterogeneous tumor antigens, immunoPET imaging is gradually changing the theranostic landscape of several types of malignancies. ImmunoPET is the method of choice for imaging specific tumor markers, immune cells, immune checkpoints, and inflammatory processes. Furthermore, the integration of immunoPET imaging in antibody drug development is of substantial significance because it provides pivotal information regarding antibody targeting abilities and distribution profiles. Herein, we present the latest immunoPET imaging strategies and their preclinical and clinical applications. We also emphasize current conjugation strategies that can be leveraged to develop next-generation immunoPET probes. Lastly, we discuss practical considerations to tune the development and translation of immunoPET imaging strategies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




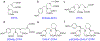














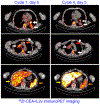

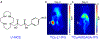






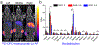
References
-
- Mankoff DA A definition of molecular imaging. J. Nucl. Med 2007, 48, 18N–21N. - PubMed
-
- Ametamey SM; Honer M; Schubiger PA Molecular imaging with PET. Chem. Rev 2008, 108, 1501–1516. - PubMed
-
- James ML; Gambhir SS A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev 2012, 92, 897–965. - PubMed
-
- Rahmim A; Lodge MA; Karakatsanis NA; Panin VY; Zhou Y; McMillan A; Cho S; Zaidi H; Casey ME; Wahl RL Dynamic whole-body PET imaging: principles, potentials and applications. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 501–518. - PubMed
-
- Scott AM; Wolchok JD; Old LJ Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

