Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn's disease
- PMID: 32202328
- PMCID: PMC7160034
- DOI: 10.1111/apt.15685
Systematic review and meta-analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn's disease
Abstract
Background: There is an increasing body of evidence showing that earlier use of biologics improves clinical outcomes in Crohn's disease (CD).
Aim: To perform a systematic review and meta-analysis to assess the impact of early biologic use in the treatment of CD.
Methods: PubMed and Embase databases were searched for English language papers and conference abstracts published through April 30, 2019. Studies were selected for inclusion if patients initiated biologics within 2 years of a CD diagnosis or if earlier biologics use (top-down) was compared with a conventional step-up strategy. Random-effects meta-analyses were conducted to compare clinical remission (CR), relapse and endoscopic healing rates between early biologic treatment (<2 years of disease duration or top-down treatment strategy) and late/conventional treatment (biologic use after >2 years of disease duration or conventional step-up treatment strategy).
Results: A total of 3069 records were identified, of which 47 references met the selection criteria for systematic review. A total of 18 471 patients were studied, with a median follow-up of 64 weeks (range 10-416). Meta-analysis found that early use of biologics was associated with higher rates of clinical remission (OR 2.10 [95% CI: 1.69-2.60], n = 2763, P < .00001), lower relapse rates (OR 0.31 [95% CI: 0.14-0.68], n = 596, P = .003) and higher mucosal healing rates (OR 2.37 [95% CI: 1.78-3.16], n = 994, P < .00001) compared with late/conventional management.
Conclusions: Early biologic treatment is associated with improved clinical outcomes in both adult and paediatric CD patients, not only in prospective clinical trials but also in real-world settings.
© 2020 John Wiley & Sons Ltd.
Figures

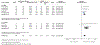
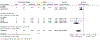
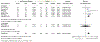
References
-
- Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn’s disease. Current drug targets. 2014;15(11):1056–1063. - PubMed
-
- Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn’s disease. Lancet (London, England). 2017;389(10080):1741–1755. - PubMed
-
- Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflammatory bowel diseases. 2002;8(4):244–250. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
