In the presence of Trypanosoma cruzi antigens, activated peripheral T lymphocytes retained in the liver induce a proinflammatory phenotypic and functional shift in intrahepatic T lymphocyte
- PMID: 32202341
- PMCID: PMC7383480
- DOI: 10.1002/JLB.3A0220-399RR
In the presence of Trypanosoma cruzi antigens, activated peripheral T lymphocytes retained in the liver induce a proinflammatory phenotypic and functional shift in intrahepatic T lymphocyte
Abstract
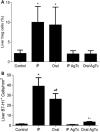
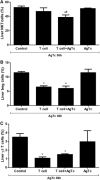
In secondary lymphoid organs, pathogen-derived and endogenous danger molecules are recognized by pattern recognition receptors, leading to adaptive proinflammatory immune responses. This conceptual rule does not apply directly to the liver, as hepatic immune cells tolerate gut-derived bacterial molecules from the flora. Therefore, the recognition of danger and proinflammatory stimuli differs between the periphery and the liver. However, the tolerant nature of the liver must be overcome in the case of infections or cancer, for example. The central paradigm is the basis for danger recognition and the balance between inflammation and tolerance in the liver. Here, we observed functional integration, with activated peripheral T lymphocytes playing a role in the induction of a proinflammatory environment in the liver in the presence of Trypanosoma cruzi antigens. When only parasite extract was orally administered, it led to the up-regulation of hepatic tolerance markers, but oral treatment plus adoptively transferred activated splenic T lymphocytes led to a proinflammatory response. Moreover, treated/recipient mice showed increased levels of TNF, IFN-γ, IL-6, and CCL2 in the liver and increased numbers of effector and/or effector memory T lymphocytes and F4/80+ cells. There was a reduction in FoxP3+ Treg cells, NKT cells, and γδ T lymphocytes with increased liver damage in the presence of activated peripheral T cells. Our results show that the induction of a proinflammatory liver response against T. cruzi danger molecules is at least partially dependent on cooperation with activated peripheral T cells.
Keywords: Trypanosoma. cruzi; hepatic T lymphocytes; hepatic immunoregulation; liver immune response; pathogen-associated molecular pattern (PAMP); peripheral T cells.
© 2020 The Authors. Journal of Leukocyte Biology published by Wiley Periodicals, Inc. on behalf of Society for Leukocyte Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Limited Foxp3+ Regulatory T Cells Response During Acute Trypanosoma cruzi Infection Is Required to Allow the Emergence of Robust Parasite-Specific CD8+ T Cell Immunity.Front Immunol. 2018 Nov 5;9:2555. doi: 10.3389/fimmu.2018.02555. eCollection 2018. Front Immunol. 2018. PMID: 30455700 Free PMC article.
-
Specific activation of CD4- CD8- double-negative T cells by Trypanosoma cruzi-derived glycolipids induces a proinflammatory profile associated with cardiomyopathy in Chagas patients.Clin Exp Immunol. 2017 Oct;190(1):122-132. doi: 10.1111/cei.12992. Epub 2017 Jul 3. Clin Exp Immunol. 2017. PMID: 28543170 Free PMC article.
-
An Animal Model of Acute and Chronic Chagas Disease With the Reticulotropic Y Strain of Trypanosoma cruzi That Depicts the Multifunctionality and Dysfunctionality of T Cells.Front Immunol. 2019 Apr 26;10:918. doi: 10.3389/fimmu.2019.00918. eCollection 2019. Front Immunol. 2019. PMID: 31105709 Free PMC article.
-
Trypanosoma cruzi-induced molecular mimicry and Chagas' disease.Curr Top Microbiol Immunol. 2005;296:89-123. doi: 10.1007/3-540-30791-5_6. Curr Top Microbiol Immunol. 2005. PMID: 16323421 Review.
-
Evasion of immune responses by Trypanosoma cruzi, the etiological agent of Chagas disease.Braz J Med Biol Res. 2011 Feb;44(2):84-90. doi: 10.1590/s0100-879x2011007500005. Epub 2011 Jan 14. Braz J Med Biol Res. 2011. PMID: 21243314 Review.
Cited by
-
The liver's dilemma: sensing real danger in a sea of PAMPs: the (arterial) sinusoidal segment theory.Front Immunol. 2025 Jan 27;15:1503063. doi: 10.3389/fimmu.2024.1503063. eCollection 2024. Front Immunol. 2025. PMID: 39931578 Free PMC article. Review.
-
In the Acute Phase of Trypanosoma cruzi Infection, Liver Lymphoid and Myeloid Cells Display an Ambiguous Phenotype Combining Pro- and Anti-Inflammatory Markers.Front Immunol. 2022 May 26;13:868574. doi: 10.3389/fimmu.2022.868574. eCollection 2022. Front Immunol. 2022. PMID: 35720410 Free PMC article.
-
After Experimental Trypanosoma cruzi Infection, Dying Hepatic CD3+TCRαβ+B220+ T Lymphocytes Are Rescued from Death by Peripheral T Cells and Become Activated.Pathogens. 2020 Aug 31;9(9):717. doi: 10.3390/pathogens9090717. Pathogens. 2020. PMID: 32878101 Free PMC article.
-
Immunological Tolerance in Liver Transplant Recipients: Putative Involvement of Neuroendocrine-Immune Interactions.Cells. 2022 Jul 29;11(15):2327. doi: 10.3390/cells11152327. Cells. 2022. PMID: 35954171 Free PMC article. Review.
-
The Liver and the Hepatic Immune Response in Trypanosoma cruzi Infection, a Historical and Updated View.Pathogens. 2021 Aug 25;10(9):1074. doi: 10.3390/pathogens10091074. Pathogens. 2021. PMID: 34578107 Free PMC article. Review.
References
-
- Matzinger P. An innate sense of danger. Ann NY Acad Sci. 2002;961:341‐342. - PubMed
-
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197‐216. - PubMed
-
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821‐832. - PubMed
-
- Kern M, Popov A, Scholz K, et al. Virally infected mouse liver endothelial cells trigger CD8+ T‐cell immunity. Gastroenterology. 2010;138:336‐346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

