Real-world assay variability between laboratories in monitoring of recombinant factor IX Fc fusion protein activity in plasma samples
- PMID: 32202380
- PMCID: PMC7318191
- DOI: 10.1111/ijlh.13189
Real-world assay variability between laboratories in monitoring of recombinant factor IX Fc fusion protein activity in plasma samples
Abstract
Introduction: Monitoring of factor IX (FIX) replacement therapy in haemophilia B relies on accurate coagulation assays. However, considerable interlaboratory variability has been reported for one-stage clotting (OSC) assays. This study aimed to evaluate the real-world, interlaboratory variability of routine FIX activity assays used in clinical haemostasis laboratories for the measurement of recombinant FIX Fc fusion protein (rFIXFc) activity.
Methods: Human FIX-depleted plasma was spiked with rFIXFc at 0.80, 0.20 or 0.05 IU/mL based on label potency. Participating laboratories tested samples using their own routine OSC or chromogenic substrate (CS) assay protocols, reagents and FIX plasma standards. Laboratories could perform more than one measurement and method, and were not fully blinded to nominal activity values.
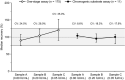
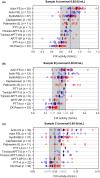
Results: A total of 142 laboratories contributed OSC results from 175 sample kits using 11 different activated partial thromboplastin time (aPTT) reagents. The median recovered FIX activity for the 0.80, 0.20 and 0.05 IU/mL samples was 0.72 IU/mL, 0.21 IU/mL and 0.060 IU/mL, respectively. Across all OSC reagents, interlaboratory variability (% CV) per aPTT reagent ranged from 9.4% to 32.1%, 8.2% to 32.6% and 12.2% to 42.0% at the 0.80, 0.20 and 0.05 IU/mL levels, respectively. CS results showed excellent median recoveries at all nominal levels (87.5% to 115.0%; n = 11) with low interlaboratory variability (CV 3.6% to 15.4%).
Conclusion: This large, real-world data set indicates that rFIXFc activity in plasma samples can be accurately measured with the majority of routine OSC and CS assay methods. Given the variation in FIX assay procedures between sites, it is important that individual laboratories qualify their in-house methods for monitoring of rFIXFc activity.
Keywords: chromogenic substrate assay; factor IX replacement therapy; haemophilia B; one-stage clotting assay; recombinant factor IX Fc fusion protein.
© 2020 Swedish Orphan Biovitrum AB. International Journal of Laboratory Hematology published by John Wiley & Sons Ltd.
Conflict of interest statement
JS: Employee of Biogen at the time of the study; consultancy fees from Sobi; ASK: Employee of Precision BioLogic; CB: Employee of Sanofi Genzyme; MW: Employee of Sobi at the time of the study; AW: Employee of Sobi at the time of the study; current employee of Sanofi.
Figures
References
-
- Iorio A, Blanchette V, Blatny J, Collins P, Fischer K, Neufeld E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15(12):2461‐2465. - PubMed
-
- Iorio A, Fischer K, Blanchette V, Rangarajan S, Young G, Morfini M. Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half‐life FIX concentrates. Thromb Haemost. 2017;117(6):1023‐1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

