Inflammatory osteolysis is regulated by site-specific ISGylation of the scaffold protein NEMO
- PMID: 32202502
- PMCID: PMC7145425
- DOI: 10.7554/eLife.56095
Inflammatory osteolysis is regulated by site-specific ISGylation of the scaffold protein NEMO
Abstract
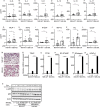
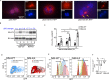
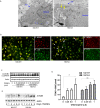
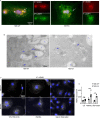
Inflammatory osteolysis is governed by exacerbated osteoclastogenesis. Ample evidence points to central role of NF-κB in such pathologic responses, yet the precise mechanisms underpinning specificity of these responses remain unclear. We propose that motifs of the scaffold protein IKKγ/NEMO partly facilitate such functions. As proof-of-principle, we used site-specific mutagenesis to examine the role of NEMO in mediating RANKL-induced signaling in mouse bone marrow macrophages, known as osteoclast precursors. We identified lysine (K)270 as a target regulating RANKL signaling as K270A substitution results in exuberant osteoclastogenesis in vitro and murine inflammatory osteolysis in vivo. Mechanistically, we discovered that K270A mutation disrupts autophagy, stabilizes NEMO, and elevates inflammatory burden. Specifically, K270A directly or indirectly hinders binding of NEMO to ISG15, a ubiquitin-like protein, which we show targets the modified proteins to autophagy-mediated lysosomal degradation. Taken together, our findings suggest that NEMO serves as a toolkit to fine-tune specific signals in physiologic and pathologic conditions.
Keywords: ISG15; NEMO; RANKL; human biology; medicine; mouse; nk-kb; osteoclast; osteolysis.
Plain language summary
The human skeleton contains over 200 bones that together act as an internal framework for the body. Over our lifetime, the body constantly removes older bone tissue from the skeleton and replaces it with new bone tissue. This “bone remodeling” also controls how bones are repaired after being damaged by injuries, disease or normal wear and tear. Cells known as osteoclasts are responsible for breaking down old bone tissue and participate in repairing damaged bone. A cellular pathway known as NF-kB signaling stimulates other cells called “bone marrow macrophages” to become osteoclasts. A certain level of NF-kB signaling is required to maintain a healthy skeleton. However, under certain inflammatory conditions, the level of NF-kB signaling becomes too high causing hyperactive osteoclasts to accumulate and inflict severe bone breakdown. This abnormal osteoclast activity leads to eroded and fragile bones and joints, as is the case in diseases such as rheumatoid arthritis and osteoporosis. Previous studies have shown that a protein called NEMO is a core component of the NF-kB signal pathway, but the precise role of NEMO in the diseased response remained unclear. Adapala, Swarnkar, Arra et al. have now used site-directed mutagenesis approach to study the role of NEMO in bone marrow macrophages in mice. The experiments showed that one specific site within the NEMO protein, referred to as lysine 270, is crucial for its role in controlling osteoclasts and the breakdown of bone tissue. Mutating NEMO at lysine 270 led to uncontrolled NF-kB signaling in the bone marrow macrophages. Further experiments showed that lysine 270 served as a sensor to allow NEMO to bind another protein called ISG15, which in turn helped to decrease NF-kB signaling and slow down the erosion of the bone. These findings suggest that site-specific targeting of NEMO, rather than inhibiting the whole NF-kB pathway, may help to reduce the symptoms of bone disease while maintaining the beneficial roles of this essential pathway. However, additional research is required to identify NEMO sites responsible for controlling the inflammatory component.
© 2020, Adapala et al.
Conflict of interest statement
NA, GS, MA, JS, GM, KK, YA No competing interests declared
Figures






Similar articles
-
Impediment of NEMO oligomerization inhibits osteoclastogenesis and osteolysis.J Cell Biochem. 2009 Dec 15;108(6):1337-45. doi: 10.1002/jcb.22364. J Cell Biochem. 2009. PMID: 19830703 Free PMC article.
-
Lysine392, a K63-linked ubiquitination site in NEMO, mediates inflammatory osteoclastogenesis and osteolysis.J Orthop Res. 2012 Apr;30(4):554-60. doi: 10.1002/jor.21555. Epub 2011 Sep 12. J Orthop Res. 2012. PMID: 21913221 Free PMC article.
-
Polyubiquitination events mediate polymethylmethacrylate (PMMA) particle activation of NF-kappaB pathway.J Biol Chem. 2011 Jul 8;286(27):23735-41. doi: 10.1074/jbc.M111.223669. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566132 Free PMC article.
-
Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption.Front Immunol. 2018 Sep 28;9:2263. doi: 10.3389/fimmu.2018.02263. eCollection 2018. Front Immunol. 2018. PMID: 30323820 Free PMC article. Review.
-
Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis.Front Immunol. 2023 Oct 4;14:1274679. doi: 10.3389/fimmu.2023.1274679. eCollection 2023. Front Immunol. 2023. PMID: 37860014 Free PMC article. Review.
Cited by
-
The diverse repertoire of ISG15: more intricate than initially thought.Exp Mol Med. 2022 Nov;54(11):1779-1792. doi: 10.1038/s12276-022-00872-3. Epub 2022 Nov 1. Exp Mol Med. 2022. PMID: 36319753 Free PMC article. Review.
-
Glutamine metabolism modulates chondrocyte inflammatory response.Elife. 2022 Aug 2;11:e80725. doi: 10.7554/eLife.80725. Elife. 2022. PMID: 35916374 Free PMC article.
-
Liquid phase separation of NEMO induced by polyubiquitin chains activates NF-κB.Mol Cell. 2022 Jul 7;82(13):2415-2426.e5. doi: 10.1016/j.molcel.2022.03.037. Epub 2022 Apr 26. Mol Cell. 2022. PMID: 35477005 Free PMC article.
-
STING-dependent interferon signatures restrict osteoclast differentiation and bone loss in mice.Proc Natl Acad Sci U S A. 2023 Apr 11;120(15):e2210409120. doi: 10.1073/pnas.2210409120. Epub 2023 Apr 6. Proc Natl Acad Sci U S A. 2023. PMID: 37023130 Free PMC article.
-
ISGylation of NF-κBp65 by SCFFBXL19 E3 Ligase Diminishes Endothelial Inflammation.Arterioscler Thromb Vasc Biol. 2023 May;43(5):674-683. doi: 10.1161/ATVBAHA.122.318894. Epub 2023 Mar 30. Arterioscler Thromb Vasc Biol. 2023. PMID: 36994728 Free PMC article.
References
-
- Abu-Amer Y, Faccio R. Therapeutic approaches in bone pathogeneses: targeting the IKK/NF-κB Axis. Future Rheumatology. 2006;1:133–146. doi: 10.2217/17460816.1.1.133. - DOI
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AR054326/NH/NIH HHS/United States
- R01 AR064755/AR/NIAMS NIH HHS/United States
- R21 AR077226/AR/NIAMS NIH HHS/United States
- AR064755/NH/NIH HHS/United States
- R01 AR068972/AR/NIAMS NIH HHS/United States
- 85160/Shriners Hospital for Children
- AR049192/NH/NIH HHS/United States
- 86200/Shriners Hospitals for Children
- 85160/Shriners Hospitals for Children
- AR075860/NH/NIH HHS/United States
- P30 AR057235/AR/NIAMS NIH HHS/United States
- AR077226/NH/NIH HHS/United States
- R01 AR054326/AR/NIAMS NIH HHS/United States
- R01 AR072623/AR/NIAMS NIH HHS/United States
- AR057235/NH/NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- R01 AR049192/AR/NIAMS NIH HHS/United States
- T32 GM007200/GM/NIGMS NIH HHS/United States
- R01 AR075860/AR/NIAMS NIH HHS/United States
- AR068972/NH/NIH HHS/United States
- 86200/Shriners Hospital For Children
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

