Hippocampal Synaptic Plasticity, Spatial Memory, and Neurotransmitter Receptor Expression Are Profoundly Altered by Gradual Loss of Hearing Ability
- PMID: 32202614
- PMCID: PMC7325716
- DOI: 10.1093/cercor/bhaa061
Hippocampal Synaptic Plasticity, Spatial Memory, and Neurotransmitter Receptor Expression Are Profoundly Altered by Gradual Loss of Hearing Ability
Abstract
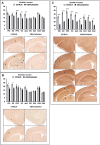
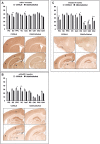
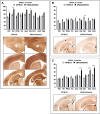
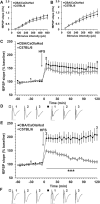
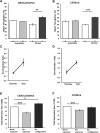
Sensory information comprises the substrate from which memories are created. Memories of spatial sensory experience are encoded by means of synaptic plasticity in the hippocampus. Hippocampal dependency on sensory information is highlighted by the fact that sudden and complete loss of a sensory modality results in an impairment of hippocampal function that persists for months. Effects are accompanied by extensive changes in the expression of neurotransmitter receptors in cortex and hippocampus, consistent with a substantial adaptive reorganization of cortical function. Whether gradual sensory loss affects hippocampal function is unclear. Progressive age-dependent hearing loss (presbycusis) is a risk factor for cognitive decline. Here, we scrutinized C57BL/6 mice that experience hereditary and cumulative deafness starting in young adulthood. We observed that 2-4 months postnatally, increases in the cortical and hippocampal expression of GluN2A and GluN2B subunits of the N-methyl-D-aspartate receptor occurred compared to control mice that lack sensory deficits. Furthermore, GABA and metabotropic glutamate receptor expression were significantly altered. Hippocampal synaptic plasticity was profoundly impaired and mice exhibited significant deficits in spatial memory. These data show that during cortical adaptation to cumulative loss of hearing, plasticity-related neurotransmitter expression is extensively altered in the cortex and hippocampus. Furthermore, cumulative sensory loss compromises hippocampal function.
Keywords: C57BL/6; GABA; NMDA receptor; hearing loss; hippocampus; metabotropic glutamate receptor.
© The Author(s) 2020. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- André ME, Manahan-Vaughan D. 2014. Spatial olfactory learning facilitates long-term depression in the hippocampus. Hippocampus. 23:963–968. - PubMed
-
- Attems J, Walker L, Jellinger KA. 2015. Olfaction and aging: a mini-review. Gerontology. 61(6):485–490. - PubMed
-
- Berberich S, Jensen V, Hvalby O, Seeburg PH, Köhr G. 2007. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 52:77–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

