Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells
- PMID: 32203008
- PMCID: PMC7141938
- DOI: 10.2450/2020.0256-19
Decoding the metabolic landscape of pathophysiological stress-induced cell death in anucleate red blood cells
Abstract
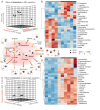
Background: In response to stress, anucleate red blood cells (RBCs) can undergo a process of atypical cell death characterised by intracellular Ca2+ accumulation and phosphatidylserine (PS) externalisation. Here we studied alterations in RBC metabolism, a critical contributor to their capacity to survive environmental challenges, during this process.
Materials and methods: Metabolomics analyses of RBCs and supernatants, using ultra-high-pressure liquid chromatography coupled to mass spectrometry, were performed after in vitro exposure of RBCs to different pathophysiological cell stressors, including starvation, extracellular hypertonicity, hyperthermia, and supraphysiological ionic stress. Cell death was examined by flow cytometry.
Results: Our data show that artificially enhancing RBC cytosolic Ca2+ influx significantly enhanced purine oxidation and strongly affected cellular bioenergetics by reducing glycolysis. Depleting extracellular Ca2+ curtailed starvation-induced cell death, an effect paralleled by the activation of compensatory pathways such as the pentose phosphate pathway, carboxylic acid metabolism, increased pyruvate to lactate ratios (methemoglobin reductase activation), one-carbon metabolism (protein-damage repair) and glutathione synthesis; RBCs exposed to hypertonic shock displayed a similar metabolic profile. Furthermore, cell stress promoted lipid remodelling as reflected by the levels of free fatty acids, acyl-carnitines and CoA precursors. Notably, RBC PS exposure, independently of the stressor, showed significant correlation with the levels of free fatty acids, glutamate, cystine, spermidine, tryptophan, 5-oxoproline, lactate, and hypoxanthine.
Discussion: In conclusion, different cell death-inducing pathophysiological stressors, encountered in various clinical conditions, result in differential RBC metabolic phenotypes, only partly explained by intracellular Ca2+ levels and ATP availability.
Conflict of interest statement
Though unrelated to the contents of the manuscript, the authors declare that AD and TN are founder of Omix Technologies Inc. and Altis Bioscience LLC. All the other authors have no conflicts of interest to disclose.
Figures




Comment in
-
"Valar morghulis": all red cells must die.Blood Transfus. 2020 Mar;18(2):83-85. doi: 10.2450/2020.0028-20. Epub 2020 Mar 17. Blood Transfus. 2020. PMID: 32203010 Free PMC article. No abstract available.
References
-
- Lang E, Qadri SM, Lang F. Killing me softly - suicidal erythrocyte death. Int J Biochem Cell Biol. 2012;44:1236–43. - PubMed
-
- Qadri SM, Bissinger R, Solh Z, Oldenborg PA. Eryptosis in health and disease: a paradigm shift towards understanding the (patho) physiological implications of programmed cell death of erythrocytes. Blood Rev. 2017;31:349–61. - PubMed
-
- Pretorius E, du Plooy JN, Bester J. A comprehensive review on eryptosis. Cell Physiol Biochem. 2016;39:1977–2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
