Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain
- PMID: 32203087
- PMCID: PMC7470811
- DOI: 10.1038/s41401-020-0394-6
Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain
Abstract
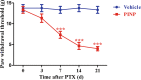
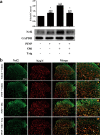
Paclitaxel-induced neuropathic pain (PINP) is refractory to currently used analgesics. Previous studies show a pivotal role of oxidative stress in PINP. Because the nuclear factor erythroid-2-related factor 2 (Nrf2) has been considered as the critical regulator of endogenous antioxidant defense, we here explored whether activation of Nrf2 could attenuate PINP. A rat model of PINP was established by intraperitoneal injection of paclitaxel (2 mg/kg) every other day with a final cumulative dose of 8 mg/kg. Hind paw withdrawal thresholds (PWTs) in response to von Frey filament stimuli were used to assess mechanical allodynia. We showed that a single dose of Nrf2 activator, oltipraz (10, 50, and 100 mg/kg), dose-dependently attenuated established mechanical allodynia, whereas repeated injection of oltipraz (100 mg· kg-1· d-1, i.p. from d 14 to d 18) almost abolished the mechanical allodynia in PINP rats. The antinociceptive effect of oltipraz was blocked by pre-injection of Nrf2 inhibitor trigonelline (20 mg/kg, i.p.). Early treatment with oltipraz (100 mg· kg-1· d-1, i.p. from d 0 to d 6) failed to prevent the development of the PINP, but delayed its onset. Western blot and immunofluorescence analysis revealed that the expression levels of Nrf2 and HO-1 were significantly upregulated in the spinal cord of PINP rats. Repeated injection of oltipraz caused further elevation of the expression levels of Nrf2 and HO-1 in the spinal cord of PINP rats, which was reversed by pre-injection of trigonelline. These results demonstrate that oltipraz ameliorates PINP via activating Nrf2/HO-1-signaling pathway in the spinal cord.
Keywords: HO-1; Nrf2; neuropathic pain; oltipraz; oxidative stress; paclitaxel.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

