It does not always take two to tango: "Syntrophy" via hydrogen cycling in one bacterial cell
- PMID: 32203116
- PMCID: PMC7242416
- DOI: 10.1038/s41396-020-0627-1
It does not always take two to tango: "Syntrophy" via hydrogen cycling in one bacterial cell
Abstract
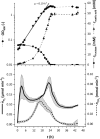
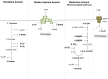
Interspecies hydrogen transfer in anoxic ecosystems is essential for the complete microbial breakdown of organic matter to methane. Acetogenic bacteria are key players in anaerobic food webs and have been considered as prime candidates for hydrogen cycling. We have tested this hypothesis by mutational analysis of the hydrogenase in the model acetogen Acetobacterium woodii. Hydrogenase-deletion mutants no longer grew on H2 + CO2 or organic substrates such as fructose, lactate, or ethanol. Heterotrophic growth could be restored by addition of molecular hydrogen to the culture, indicating that hydrogen is an intermediate in heterotrophic growth. Indeed, hydrogen production from fructose was detected in a stirred-tank reactor. The mutant grew well on organic substrates plus caffeate, an alternative electron acceptor that does not require molecular hydrogen but NADH as reductant. These data are consistent with the notion that molecular hydrogen is produced from organic substrates and then used as reductant for CO2 reduction. Surprisingly, hydrogen cycling in A. woodii is different from the known modes of interspecies or intraspecies hydrogen cycling. Our data are consistent with a novel type of hydrogen cycling that connects an oxidative and reductive metabolic module in one bacterial cell, "intracellular syntrophy."
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Novelli PC, Lang PM, Masarie KA, Hurst DF, Myers R, Elkins JW. Molecular hydrogen in the troposphere: Global distribution and budget. J Geophys Res. 1999;104:30427–44.
-
- Schink B. Synergistic interactions in the microbial world. Antonie Van Leeuwenhoek. 2002;81:257–61. - PubMed
-
- Odom JM, Peck HD., Jr Hydrogenase, electron-transfer proteins, and energy coupling in the sulfate-reducing bacteria Desulfovibrio. Annu Rev Microbiol. 1984;38:551–92. - PubMed

