Immunomodulatory effects of pevonedistat, a NEDD8-activating enzyme inhibitor, in chronic lymphocytic leukemia-derived T cells
- PMID: 32203139
- PMCID: PMC8288064
- DOI: 10.1038/s41375-020-0794-0
Immunomodulatory effects of pevonedistat, a NEDD8-activating enzyme inhibitor, in chronic lymphocytic leukemia-derived T cells
Abstract
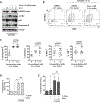
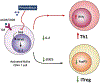
Novel targeted agents used in therapy of lymphoid malignancies, such as inhibitors of B-cell receptor-associated kinases, are recognized to have complex immune-mediated effects. NEDD8-activating enzyme (NAE) has been identified as a tractable target in chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma. We and others have shown that pevonedistat (TAK-924), a small-molecule inhibitor of NAE, abrogates NF-κB signaling in malignant B cells. However, NF-κB pathway activity is indispensable in immune response, and T-cell function is altered in patients with CLL. Using T cells derived from patients with CLL, we demonstrate that although targeting NAE results in markedly differential expression of NF-κB-regulated genes and downregulation of interleukin (IL)-2 signaling during T-cell activation, T cells evade apoptosis. Meanwhile, NAE inhibition favorably modulates polarization of T cells in vitro, with decreased Treg differentiation and a shift toward TH1 phenotype, accompanied by increased interferon-γ production. These findings were recapitulated in vivo in immunocompetent mouse models. T cells exposed to pevonedistat in washout experiments, informed by its human pharmacokinetic profile, recover NAE activity, and maintain their response to T-cell receptor stimulation and cytotoxic potential. Our data shed light on the potential immune implications of targeting neddylation in CLL and lymphoid malignancies.
Figures



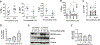

References
-
- Kittai AS, Lunning M, Danilov AV. Relevance of prognostic factors in the era of targeted therapies in CLL. Curr Hematol Malig Rep. 2019;14:302–9. - PubMed
-
- Shindiapina P, Brown JR, Danilov AV. A new hope: novel therapeutic approaches to treatment of chronic lymphocytic leukaemia with defects in TP53. Br J Haematol. 2014;167:149–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

