Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury
- PMID: 32203170
- PMCID: PMC7429834
- DOI: 10.1038/s41418-020-0528-x
Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury
Abstract
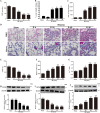
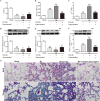
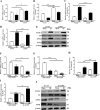
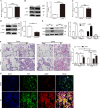
Acute lung injury (ALI) is a life-threatening disorder with high rates of morbidity and mortality. Reactive oxygen species and epithelial apoptosis are involved in the pathogenesis of acute lung injury. Ferroptosis, an iron-dependent non-apoptotic form of cell death, mediates its effects in part by promoting the accumulation of reactive oxygen species. The inhibition of ferroptosis decreases clinical symptoms in experimental models of ischemia/reperfusion-induced renal failure and heart injury. This study investigated the roles of inhibitor of apoptosis-stimulating protein of p53 (iASPP) and Nrf2 in ferroptosis and their potential therapeutic effects in intestinal ischemia/reperfusion-induced acute lung injury. Intestinal ischemia/reperfusion-induced ALI was induced in wild-type and Nrf2-/- mice. The mice were treated with erastin followed by liproxstatin-1. Ferroptosis-related factors in mice with ischemia/reperfusion-induced acute lung injury or in mouse lung epithelial-2 cells with hypoxia/regeneration (HR)-induced ALI were measured by western blotting, real-time PCR, and immunofluorescence. Ferroptosis contributed to intestinal ischemia/reperfusion-induced ALI in vivo. iASPP inhibited ferroptosis and alleviated intestinal ischemia/reperfusion-induced acute lung injury, and iASPP-mediated protection against ischemia/reperfusion-induced ALI was dependent on Nrf2 signaling. HR-induced acute lung injury enhanced ferroptosis in vitro in mouse lung epithelial-2 cells, and ferroptosis was modulated after the enhancement of intestinal ischemia/reperfusion in Nrf2-/- mice. iASPP mediated its protective effects against acute lung injury through the Nrf2/HIF-1/TF signaling pathway. Ferroptosis contributes to intestinal ischemia/reperfusion-induced ALI, and iASPP treatment inhibits ferroptosis in part via Nrf2. These findings indicate the therapeutic potential of iASPP for treating ischemia/reperfusion-induced ALI.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

