HPV-mediated nuclear export of HP1γ drives cervical tumorigenesis by downregulation of p53
- PMID: 32203172
- PMCID: PMC7429875
- DOI: 10.1038/s41418-020-0520-5
HPV-mediated nuclear export of HP1γ drives cervical tumorigenesis by downregulation of p53
Abstract
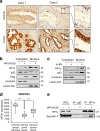
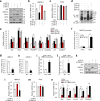
E6 oncoprotein derived from high-risk human papillomavirus (HPV) drives the development of cervical cancer through p53 degradation. Because cervical cancer therapies to inactivate HPV or E6 protein are not available, alternative strategies are required. Here, we show that HPV-mediated nuclear export of human heterochromatin protein 1γ (HP1γ) reduces the stability of p53 through UBE2L3-mediated p53 polyubiquitination during cervical cancer progression. In general, HP1 plays a key role in heterochromatin formation and transcription in the nucleus. However, our immunostaining data showed that the majority of HP1γ is localized in the cytoplasm in HPV-mediated cervical cancer. We found that HPV E6 protein drives unusual nuclear export of HP1γ through the interaction between the NES sequence of HP1γ and exportin-1. The mutation of the NES sequence in HP1γ led to nuclear retention of HP1γ and reduced cervical cancer cell growth and tumor generation. We further discovered that HP1γ directly suppresses the expression of UBE2L3 which drives E6-mediated proteasomal degradation of p53 in cervical cancer. Downregulation of UBE2L3 by overexpression of HP1γ suppressed UBE2L3-dependent p53 degradation-promoting apoptosis of cervical cancer cells. Our findings propose a useful strategy to overcome p53 degradation in cervical cancer through the blockage of nuclear export of HP1γ.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
HP1γ Sensitizes Cervical Cancer Cells to Cisplatin through the Suppression of UBE2L3.Int J Mol Sci. 2020 Aug 19;21(17):5976. doi: 10.3390/ijms21175976. Int J Mol Sci. 2020. PMID: 32825184 Free PMC article.
-
Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation.Biochemistry. 2000 Nov 14;39(45):13916-24. doi: 10.1021/bi001489b. Biochemistry. 2000. PMID: 11076533
-
Crucial role of TSC-22 in preventing the proteasomal degradation of p53 in cervical cancer.PLoS One. 2012;7(8):e42006. doi: 10.1371/journal.pone.0042006. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870275 Free PMC article.
-
The role of TP53 in Cervical carcinogenesis.Hum Mutat. 2003 Mar;21(3):307-12. doi: 10.1002/humu.10178. Hum Mutat. 2003. PMID: 12619117 Review.
-
Hyperactivating p53 in Human Papillomavirus-Driven Cancers: A Potential Therapeutic Intervention.Mol Diagn Ther. 2022 May;26(3):301-308. doi: 10.1007/s40291-022-00583-5. Epub 2022 Apr 5. Mol Diagn Ther. 2022. PMID: 35380358 Free PMC article. Review.
Cited by
-
The p53 family member p73 in the regulation of cell stress response.Biol Direct. 2021 Nov 8;16(1):23. doi: 10.1186/s13062-021-00307-5. Biol Direct. 2021. PMID: 34749806 Free PMC article. Review.
-
A functional crosstalk between the H3K9 methylation writers and their reader HP1 in safeguarding embryonic stem cell identity.Stem Cell Reports. 2023 Sep 12;18(9):1775-1792. doi: 10.1016/j.stemcr.2023.08.004. Stem Cell Reports. 2023. PMID: 37703822 Free PMC article.
-
Induction of the p21/CDK6 pathway and alteration of the immune microenvironment by the stem cell marker CBX3 in melanoma.Stem Cell Res Ther. 2025 Feb 11;16(1):63. doi: 10.1186/s13287-025-04179-8. Stem Cell Res Ther. 2025. PMID: 39934923 Free PMC article.
-
Viral oncogenesis in cancer: from mechanisms to therapeutics.Signal Transduct Target Ther. 2025 May 12;10(1):151. doi: 10.1038/s41392-025-02197-9. Signal Transduct Target Ther. 2025. PMID: 40350456 Free PMC article. Review.
-
Infection of Brain Organoids and 2D Cortical Neurons with SARS-CoV-2 Pseudovirus.Viruses. 2020 Sep 8;12(9):1004. doi: 10.3390/v12091004. Viruses. 2020. PMID: 32911874 Free PMC article.
References
-
- Holowaty P, Miller AB, Rohan T, To T. Natural dysplasia of the uterine cervix. J Nat Cancer Inst. 1999;91:252–8. - PubMed
-
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–32. - PubMed
-
- Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298:743–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

