Initial experience from a renal genetics clinic demonstrates a distinct role in patient management
- PMID: 32203225
- PMCID: PMC7272321
- DOI: 10.1038/s41436-020-0772-y
Initial experience from a renal genetics clinic demonstrates a distinct role in patient management
Erratum in
-
Correction: Initial experience from a renal genetics clinic demonstrates a distinct role in patient management.Genet Med. 2021 Oct;23(10):2017-2019. doi: 10.1038/s41436-020-01000-0. Genet Med. 2021. PMID: 33024316 Free PMC article. No abstract available.
Abstract
Purpose: A Renal Genetics Clinic (RGC) was established to optimize diagnostic testing, facilitate genetic counseling, and direct clinical management.
Methods: Retrospective review of patients seen over a two-year period in the RGC.
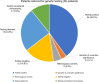
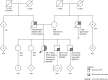
Results: One hundred eleven patients (mean age: 39.9 years) were referred to the RGC: 65 for genetic evaluation, 19 for management of a known genetic disease, and 18 healthy living kidney donors (LKDs) and their 9 related transplant candidates for screening. Forty-three patients underwent genetic testing with a diagnosis in 60% of patients including 9 with Alport syndrome, 7 with autosomal dominant polycystic kidney disease (ADPKD), 2 with genetic focal segmental glomerulosclerosis (FSGS), 2 with PAX2-mediated CAKUT, and 1 each with autosomal recessive polycystic kidney disease (ARPKD), Dent, Frasier, Gordon, Gitelman, and Zellweger syndromes. Four of 18 LKDs were referred only for APOL1 screening. For the remaining 14 LKDs, their transplant candidates were first tested to establish a genetic diagnosis. Five LKDs tested negative for the familial genetic variant, four were positive for their familial variant. In five transplant candidates, a genetic variant could not be identified.
Conclusion: An RGC that includes genetic counseling enhances care of renal patients by improving diagnosis, directing management, affording presymptomatic family focused genetic counseling, and assisting patients and LKDs to make informed decisions.
Keywords: comprehensive renal panel; genetic counseling; genetic screening; next-generation sequencing; presymptomatic testing.
Conflict of interest statement
C.P.T. and R.J.S. help develop KidneySeq™ at the University of Iowa as a comprehensive clinical genetic testing panel to evaluate patients with various renal phenotypes. M.H. has received financial support from Sanofi Genzyme for Advisory Board activity. He has received research support from Protalix Biotherapeutics and Idorsia Pharmaceuticals Ltd. The institution received funds to support the research study. The other authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

