Protein transmission in neurodegenerative disease
- PMID: 32203399
- PMCID: PMC9242841
- DOI: 10.1038/s41582-020-0333-7
Protein transmission in neurodegenerative disease
Abstract
Most neurodegenerative diseases are characterized by the intracellular or extracellular aggregation of misfolded proteins such as amyloid-β and tau in Alzheimer disease, α-synuclein in Parkinson disease, and TAR DNA-binding protein 43 in amyotrophic lateral sclerosis. Accumulating evidence from both human studies and disease models indicates that intercellular transmission and the subsequent templated amplification of these misfolded proteins are involved in the onset and progression of various neurodegenerative diseases. The misfolded proteins that are transferred between cells are referred to as 'pathological seeds'. Recent studies have made exciting progress in identifying the characteristics of different pathological seeds, particularly those isolated from diseased brains. Advances have also been made in our understanding of the molecular mechanisms that regulate the transmission process, and the influence of the host cell on the conformation and properties of pathological seeds. The aim of this Review is to summarize our current knowledge of the cell-to-cell transmission of pathological proteins and to identify key questions for future investigation.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
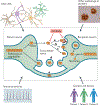

References
-
- Glenner GG & Wong CW Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun 120, 885–890 (1984). - PubMed
-
- Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006). - PubMed
-
- DiFiglia M et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

