11-Oxygenated androgens in health and disease
- PMID: 32203405
- PMCID: PMC7881526
- DOI: 10.1038/s41574-020-0336-x
11-Oxygenated androgens in health and disease
Abstract
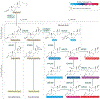
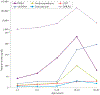
The adrenal gland is a source of sex steroid precursors, and its activity is particularly relevant during fetal development and adrenarche. Following puberty, the synthesis of androgens by the adrenal gland has been considered of little physiologic importance. Dehydroepiandrosterone (DHEA) and its sulfate, DHEAS, are the major adrenal androgen precursors, but they are biologically inactive. The second most abundant unconjugated androgen produced by the human adrenals is 11β-hydroxyandrostenedione (11OHA4). 11-Ketotestosterone, a downstream metabolite of 11OHA4 (which is mostly produced in peripheral tissues), and its 5α-reduced product, 11-ketodihydrotestosterone, are bioactive androgens, with potencies equivalent to those of testosterone and dihydrotestosterone. These adrenal-derived androgens all share an oxygen atom on carbon 11, so we have collectively termed them 11-oxyandrogens. Over the past decade, these androgens have emerged as major components of several disorders of androgen excess, such as congenital adrenal hyperplasia, premature adrenarche and polycystic ovary syndrome, as well as in androgen-dependent tumours, such as castration-resistant prostate cancer. Moreover, in contrast to the more extensively studied, traditional androgens, circulating concentrations of 11-oxyandrogens do not demonstrate an age-dependent decline. This Review focuses on the rapidly expanding knowledge regarding the implications of 11-oxyandrogens in human physiology and disease.
Figures
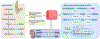
Similar articles
-
Profiling adrenal 11β-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC2-MS/MS quantification of 11β-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone.J Steroid Biochem Mol Biol. 2017 Feb;166:54-67. doi: 10.1016/j.jsbmb.2016.06.009. Epub 2016 Jun 21. J Steroid Biochem Mol Biol. 2017. PMID: 27345701
-
A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids.Mol Cell Endocrinol. 2017 Feb 5;441:76-85. doi: 10.1016/j.mce.2016.08.014. Epub 2016 Aug 9. Mol Cell Endocrinol. 2017. PMID: 27519632 Review.
-
Circulating 11-oxygenated androgens across species.J Steroid Biochem Mol Biol. 2019 Jun;190:242-249. doi: 10.1016/j.jsbmb.2019.04.005. Epub 2019 Apr 5. J Steroid Biochem Mol Biol. 2019. PMID: 30959151 Free PMC article.
-
The clinical and biochemical significance of 11-oxygenated androgens in human health and disease.Eur J Endocrinol. 2023 Apr 5;188(4):R98-R109. doi: 10.1093/ejendo/lvad047. Eur J Endocrinol. 2023. PMID: 37041725 Review.
-
Clinical significance of 11-oxygenated androgens.Curr Opin Endocrinol Diabetes Obes. 2017 Jun;24(3):252-259. doi: 10.1097/MED.0000000000000334. Curr Opin Endocrinol Diabetes Obes. 2017. PMID: 28234803 Free PMC article.
Cited by
-
Normal and Premature Adrenarche.Endocr Rev. 2021 Nov 16;42(6):783-814. doi: 10.1210/endrev/bnab009. Endocr Rev. 2021. PMID: 33788946 Free PMC article. Review.
-
Update on adrenarche.Curr Opin Pediatr. 2020 Aug;32(4):574-581. doi: 10.1097/MOP.0000000000000928. Curr Opin Pediatr. 2020. PMID: 32692055 Free PMC article. Review.
-
Changes in melatonin and sex steroid hormone production among men as a result of rotating night shift work - the HORMONIT study.Scand J Work Environ Health. 2022 Jan 1;48(1):41-51. doi: 10.5271/sjweh.3991. Epub 2021 Oct 8. Scand J Work Environ Health. 2022. PMID: 34623452 Free PMC article.
-
Salivary androgens in adolescence and their value as a marker of puberty: results from the SCAMP cohort.Endocr Connect. 2023 Nov 8;12(12):e230084. doi: 10.1530/EC-23-0084. Print 2023 Dec 1. Endocr Connect. 2023. PMID: 37800674 Free PMC article.
-
11-Oxygenated androgens are not secreted by the human ovary: in-vivo data from four different cases of hyperandrogenism.Eur J Endocrinol. 2022 Nov 23;187(6):K47-K53. doi: 10.1530/EJE-22-0518. Print 2022 Dec 1. Eur J Endocrinol. 2022. PMID: 36239921 Free PMC article.
References
-
- White PC & Speiser PW Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. Rev 21, 245–291 (2000). - PubMed
-
- Doberne Y, Levine LS & New MI Elevated urinary testosterone and androstanediol in precocious adrenarche. Pediatric Res. 9, 794–797 (1975). - PubMed
-
- Korth-Schutz S, Levine LS & New MI Evidence for the adrenal source of androgens in precocious adrenarche. Acta Endocrinol. 82, 342–352 (1976). - PubMed
-
- Azziz R, Black V, Hines GA, Fox LM & Boots LR Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic–pituitary–adrenal axis. J. Clin. Endocrinol. Metab 83, 2317–2323 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

