The Role of Torsin AAA+ Proteins in Preserving Nuclear Envelope Integrity and Safeguarding Against Disease
- PMID: 32204310
- PMCID: PMC7175109
- DOI: 10.3390/biom10030468
The Role of Torsin AAA+ Proteins in Preserving Nuclear Envelope Integrity and Safeguarding Against Disease
Abstract
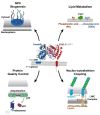
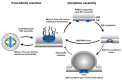
Torsin ATPases are members of the AAA+ (ATPases associated with various cellular activities) superfamily of proteins, which participate in essential cellular processes. While AAA+ proteins are ubiquitously expressed and demonstrate distinct subcellular localizations, Torsins are the only AAA+ to reside within the nuclear envelope (NE) and endoplasmic reticulum (ER) network. Moreover, due to the absence of integral catalytic features, Torsins require the NE- and ER-specific regulatory cofactors, lamina-associated polypeptide 1 (LAP1) and luminal domain like LAP1 (LULL1), to efficiently trigger their atypical mode of ATP hydrolysis. Despite their implication in an ever-growing list of diverse processes, the specific contributions of Torsin/cofactor assemblies in maintaining normal cellular physiology remain largely enigmatic. Resolving gaps in the functional and mechanistic principles of Torsins and their cofactors are of considerable medical importance, as aberrant Torsin behavior is the principal cause of the movement disorder DYT1 early-onset dystonia. In this review, we examine recent findings regarding the phenotypic consequences of compromised Torsin and cofactor activities. In particular, we focus on the molecular features underlying NE defects and the contributions of Torsins to nuclear pore complex biogenesis, as well as the growing implications of Torsins in cellular lipid metabolism. Additionally, we discuss how understanding Torsins may facilitate the study of essential but poorly understood processes at the NE and ER, and aid in the development of therapeutic strategies for dystonia.
Keywords: AAA+ ATPase; TorsinA; lipin; low-density lipoprotein (VLDL); nuclear pore complex (NPC).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Neuwald A.F., Aravind L., Spouge J.L., Koonin E.V. Aaa+: A class of chaperone-like atpases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

