Electrochemical Biosensors Based on S-Layer Proteins
- PMID: 32204503
- PMCID: PMC7147708
- DOI: 10.3390/s20061721
Electrochemical Biosensors Based on S-Layer Proteins
Abstract
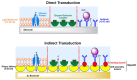
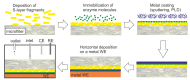
Designing and development of electrochemical biosensors enable molecule sensing and quantification of biochemical compositions with multitudinous benefits such as monitoring, detection, and feedback for medical and biotechnological applications. Integrating bioinspired materials and electrochemical techniques promote specific, rapid, sensitive, and inexpensive biosensing platforms for (e.g., point-of-care testing). The selection of biomaterials to decorate a biosensor surface is a critical issue as it strongly affects selectivity and sensitivity. In this context, smart biomaterials with the intrinsic self-assemble capability like bacterial surface (S-) layer proteins are of paramount importance. Indeed, by forming a crystalline two-dimensional protein lattice on many sensors surfaces and interfaces, the S-layer lattice constitutes an immobilization matrix for small biomolecules and lipid membranes and a patterning structure with unsurpassed spatial distribution for sensing elements and bioreceptors. This review aims to highlight on exploiting S-layer proteins in biosensor technology for various applications ranging from detection of metal ions over small organic compounds to cells. Furthermore, enzymes immobilized on the S-layer proteins allow specific detection of several vital biomolecules. The special features of the S-layer protein lattice as part of the sensor architecture enhances surface functionalization and thus may feature an innovative class of electrochemical biosensors.
Keywords: S-layer proteins; biocompatible layer; bioinspired material; biosensor; self-assembly.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
S-Layer Protein-Based Biosensors.Biosensors (Basel). 2018 Apr 11;8(2):40. doi: 10.3390/bios8020040. Biosensors (Basel). 2018. PMID: 29641511 Free PMC article.
-
Synthesis of S-layer conjugates and evaluation of their modifiability as a tool for the functionalization and patterning of technical surfaces.Molecules. 2015 May 27;20(6):9847-61. doi: 10.3390/molecules20069847. Molecules. 2015. PMID: 26023942 Free PMC article.
-
Characterization of a bacterial self-assembly surface layer protein and its application as an electrical nanobiosensor.J Nanosci Nanotechnol. 2011 Jan;11(1):402-7. doi: 10.1166/jnn.2011.3264. J Nanosci Nanotechnol. 2011. PMID: 21446464
-
Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules.Enzyme Microb Technol. 2022 May;156:110006. doi: 10.1016/j.enzmictec.2022.110006. Epub 2022 Feb 4. Enzyme Microb Technol. 2022. PMID: 35144119 Review.
-
Antifouling Strategies for Electrochemical Biosensing: Mechanisms and Performance toward Point of Care Based Diagnostic Applications.ACS Sens. 2021 Apr 23;6(4):1482-1507. doi: 10.1021/acssensors.1c00390. Epub 2021 Mar 25. ACS Sens. 2021. PMID: 33765383 Review.
Cited by
-
Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes.RSC Adv. 2022 Oct 6;12(44):28505-28518. doi: 10.1039/d2ra04947c. eCollection 2022 Oct 4. RSC Adv. 2022. PMID: 36320500 Free PMC article.
-
Advances in human papillomavirus detection for cervical cancer screening and diagnosis: challenges of conventional methods and opportunities for emergent tools.Anal Methods. 2025 Feb 13;17(7):1428-1450. doi: 10.1039/d4ay01921k. Anal Methods. 2025. PMID: 39775553 Free PMC article. Review.
-
Recent Advances in Electrochemical Biosensors for Neurodegenerative Disease Biomarkers.Biosensors (Basel). 2025 Feb 28;15(3):151. doi: 10.3390/bios15030151. Biosensors (Basel). 2025. PMID: 40136948 Free PMC article. Review.
-
Insights into the Formation of DNA-Magnetic Nanoparticle Hybrid Structures: Correlations between Morphological Characterization and Output from Magnetic Biosensor Measurements.ACS Sens. 2020 Nov 25;5(11):3510-3519. doi: 10.1021/acssensors.0c01623. Epub 2020 Nov 3. ACS Sens. 2020. PMID: 33141554 Free PMC article.
-
Gum Arabic: A Commodity with Versatile Formulations and Applications.Nanomaterials (Basel). 2025 Feb 13;15(4):290. doi: 10.3390/nano15040290. Nanomaterials (Basel). 2025. PMID: 39997853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

