Sigma receptor-induced heavy drinking in rats: Modulation by the opioid receptor system
- PMID: 32205151
- PMCID: PMC9551643
- DOI: 10.1016/j.pbb.2020.172914
Sigma receptor-induced heavy drinking in rats: Modulation by the opioid receptor system
Abstract
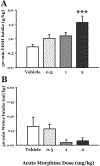
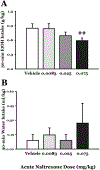
Alcohol use disorder (AUD) is a major cause of morbidity and mortality worldwide, for which new efficacious treatments are necessary. The opioid receptor system is a mediator of the rewarding effects of alcohol; in particular, while activation of μ opioid receptors enhances ethanol intake in rodents, opioid-receptor antagonists, such as naloxone and naltrexone, reduce its pleasurable and reinforcing effects, thereby decreasing alcohol. Sigma receptors (Sig-Rs) have been proposed as modulators of the effects of alcohol and, therefore, as a potential new pharmacological target for AUD. Somewhat analogously to μ opioid ligands, SigR agonists increase, while SigR antagonists decrease alcohol intake in animal models of excessive alcohol drinking. However, a potential cross-talk between these two receptor systems in relation to alcohol consumption has so far not been investigated. Here, we addressed this question pharmacologically, by testing the effects of either activating or inhibiting opioid receptors on the heavy alcohol drinking induced by chronic stimulation of SigR in alcohol-preferring rats. We found that the opioid receptor agonist morphine, which per se increases ethanol intake, at a sub-threshold dose reduces the binge-like drinking induced by the repeated treatment with the SigR agonist 1,3-di-o-tolylguanidine (DTG); conversely, the opioid receptor antagonist naltrexone, which per se reduces ethanol intake, at a sub-threshold dose potentiates the DTG-induced binge-like drinking. Our data show a cross-talk between the opioid and SigR systems relevant to the modulation of alcohol drinking, which provides important insights into the neurobiology of AUD and may lead to the development of novel therapies, either standalone or in combination.
Keywords: Addiction; Alcohol OR ethanol; Animal model; Binge; Morphine; Naltrexone; Rat; Self-administration.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest. M. Valenza is currently an employee of Epitech Group S.p.A., Saccolongo, Italy.
Figures
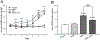
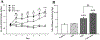
References
-
- Abbott FV, Franklin KB, Libman RB, 1986. A dose-ratio comparison of mu and kappa agonists in formalin and thermal pain. Life Sci. 39 (21), 2017–2024. - PubMed
-
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T, 2000. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97 (1), 155–170. - PubMed
-
- Altshuler HL, Phillips PE, Feinhandler DA, 1980. Alteration of ethanol self-administration by naltrexone. Life Sci. 26 (9), 679–688. - PubMed
-
- Ault DT, Werling LL, 1997. Differential modulation of NMDA-stimulated [3H]dopamine release from rat striatum by neuropeptide Y and sigma receptor ligands. Brain Res 760 (1–2), 210–217. - PubMed
-
- Ault DT, Radeff JM, Werling LL, 1998. Modulation of [3H]dopamine release from rat nucleus accumbens by neuropeptide Y may involve a sigma1-like receptor. J. Pharmacol. Exp. Ther 284 (2), 553–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

