Comparative toxicokinetics of Trans-resveratrol and its major metabolites in Harlan Sprague Dawley rats and B6C3F1/N mice following oral and intravenous administration
- PMID: 32205187
- PMCID: PMC7398575
- DOI: 10.1016/j.taap.2020.114962
Comparative toxicokinetics of Trans-resveratrol and its major metabolites in Harlan Sprague Dawley rats and B6C3F1/N mice following oral and intravenous administration
Abstract
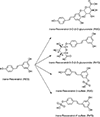
Trans-resveratrol (RES) is a naturally occurring stilbene found in numerous plants and foods. Due to its widespread human exposure and lack of toxicity and carcinogenicity data, RES was nominated to the National Toxicology Program for testing. To aid the toxicology studies, the dose, sex, and species differences in RES toxicokinetics was investigated in Harlan Sprague Dawley rats and B6C3F1/N mice following single intravenous (IV) (10 mg/kg) or oral gavage administration (312.5, 625, and 1250 mg/kg and 625, 1250, and 2500 mg/kg in rats and mice, respectively). Following IV and gavage administration, systemic exposure of RES based on AUC was trans-resveratrol-3-O-β-D-glucuronide (R3G)> > trans-resveratrol-3-sulfate (R3S) > RES in both species. Following gavage administration Tmax_predicted values were ≤ 263 min for both species and sexes. RES elimination half-life was longer in rats than mice, and shortest in male mice. Clearance was slower in mice with no apparent sex difference in both species. In both rats and mice, following gavage administration AUC increased proportionally to the dose. After gavage administration, enterohepatic recirculation of RES was observed in both rats and mice with secondary peaks occurring around 640 min in the concentration-time profiles. RES was rapidly metabolized to R3S and R3G in both species. Extensive first pass conjugation and metabolism resulted in low levels of the parent compound RES which was confirmed by the low estimates for bioavailability. The bioavailability of RES was low, ~12-31% and ~2-6% for rats and mice, respectively, with no apparent difference between sexes.
Keywords: Plasma; Resveratrol; Resveratrol-3-glucorinide; Resveratrol-3-sulfate; Toxicokinetics.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors report no declarations of interest.
Figures


References
-
- Abd El-Mohsen M, Bayele H, Kuhnle G, Gibson G, Debnam E, Kaila Srai S, Rice-Evans C, Spencer JPE, 2006. Distribution of [3H]trans-resveratrol in rat tissues following oral administration. Br. J. Nutr 96, 62–70. - PubMed
-
- Almeida L, Vaz-da-Silva M, Falcao A, Soares E, Costa R, Loureiro AI, Fernandes-Lopes C, Rocha J-F, Nunes T, Wright L, Soares-da-Silva P, 2009. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res 53 Suppl 1, S7–15. 10.1002/mnfr.200800177 - DOI - PubMed
-
- Andlauer W, Kolb J, Siebert K, Fürst P, 2000. Assessment of resveratrol bioavailability in the perfused small intestine of the rat. Drugs Exp. Clin. Res 26, 47–55. - PubMed
-
- Arichi H, Kimura Y, Okuda H, Baba K, Kozawa M, Arichi S, 1982. Effects of stilbene components of the roots of Polygonum cuspidatum Sieb. et Zucc. on lipid metabolism. Chem. Pharm. Bull. (Tokyo) 30, 1766–70. - PubMed
-
- Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM, 2002. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med 33, 387–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

