Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia
- PMID: 32205341
- PMCID: PMC7159887
- DOI: 10.1523/JNEUROSCI.1897-19.2020
Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia
Abstract
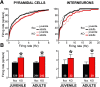
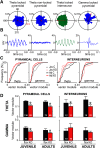
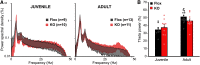
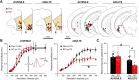
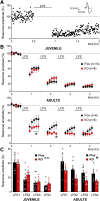
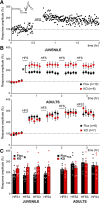
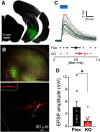
Although the etiology of schizophrenia is still unknown, it is accepted to be a neurodevelopmental disorder that results from the interaction of genetic vulnerabilities and environmental insults. Although schizophrenia's pathophysiology is still unclear, postmortem studies point toward a dysfunction of cortical interneurons as a central element. It has been suggested that alterations in parvalbumin-positive interneurons in schizophrenia are the consequence of a deficient signaling through NMDARs. Animal studies demonstrated that early postnatal ablation of the NMDAR in corticolimbic interneurons induces neurobiochemical, physiological, behavioral, and epidemiological phenotypes related to schizophrenia. Notably, the behavioral abnormalities emerge only after animals complete their maturation during adolescence and are absent if the NMDAR is deleted during adulthood. This suggests that interneuron dysfunction must interact with development to impact on behavior. Here, we assess in vivo how an early NMDAR ablation in corticolimbic interneurons impacts on mPFC and ventral hippocampus functional connectivity before and after adolescence. In juvenile male mice, NMDAR ablation results in several pathophysiological traits, including increased cortical activity and decreased entrainment to local gamma and distal hippocampal theta rhythms. In addition, adult male KO mice showed reduced ventral hippocampus-mPFC-evoked potentials and an augmented low-frequency stimulation LTD of the pathway, suggesting that there is a functional disconnection between both structures in adult KO mice. Our results demonstrate that early genetic abnormalities in interneurons can interact with postnatal development during adolescence, triggering pathophysiological mechanisms related to schizophrenia that exceed those caused by NMDAR interneuron hypofunction alone.SIGNIFICANCE STATEMENT NMDAR hypofunction in cortical interneurons has been linked to schizophrenia pathophysiology. How a dysfunction of GABAergic cortical interneurons interacts with maturation during adolescence has not been clarified yet. Here, we demonstrate in vivo that early postnatal ablation of the NMDAR in corticolimbic interneurons results in an overactive but desynchronized PFC before adolescence. Final postnatal maturation during this stage outspreads the impact of the genetic manipulation toward a functional disconnection of the ventral hippocampal-prefrontal pathway, probably as a consequence of an exacerbated propensity toward hippocampal-evoked depotentiation plasticity. Our results demonstrate a complex interaction between genetic and developmental factors affecting cortical interneurons and PFC function.
Keywords: NMDA receptor; in vivo electrophysiology; medial PFC; mice; parvalbumin interneuron; schizophrenia.
Copyright © 2020 the authors.
Figures

References
-
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KB (2004) Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 112:11096–11101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
