Telacebec for Ultrashort Treatment of Buruli Ulcer in a Mouse Model
- PMID: 32205344
- PMCID: PMC7269501
- DOI: 10.1128/AAC.00259-20
Telacebec for Ultrashort Treatment of Buruli Ulcer in a Mouse Model
Abstract
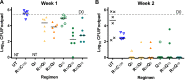
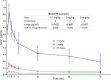
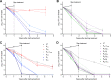
Telacebec (Q203) is a new antitubercular drug with extremely potent activity against Mycobacterium ulcerans Here, we explored the treatment-shortening potential of Q203 alone or in combination with rifampin (RIF) in a mouse footpad infection model. The first study compared Q203 at 5 and 10 mg/kg doses alone and with rifampin. Q203 alone rendered most mouse footpads culture negative in 2 weeks. Combining Q203 with rifampin resulted in a relapse-free cure 24 weeks after completing 2 weeks of treatment, compared to a 25% relapse rate in mice receiving RIF with clarithromycin, the current standard of care, for 4 weeks. The second study explored the dose-ranging activity of Q203 alone and with RIF, including the extended activity of Q203 after treatment discontinuation. The bactericidal activity of Q203 persisted for ≥ 4 weeks beyond the last dose. All mice receiving just 1 week of Q203 at 2 to 10 mg/kg were culture negative 4 weeks after stopping treatment. Mice receiving 2 weeks of Q203 at 0.5, 2, and 10 mg/kg were culture negative 4 weeks after treatment. RIF did not increase the efficacy of Q203. A pharmacokinetics substudy revealed that Q203 doses of 2 to 10 mg/kg in mice produce plasma concentrations similar to those produced by 100 to 300 mg doses in humans, with no adverse effect of RIF on Q203 concentrations. These results indicate the extraordinary potential of Q203 to reduce the duration of treatment necessary for a cure to ≤ 1 week (or 5 doses of 2 to 10 mg/kg) in our mouse footpad infection model and warrant further evaluation of Q203 in clinical trials.
Keywords: Buruli ulcer; Mycobacterium ulcerans; Q203; Telacebec.
Copyright © 2020 American Society for Microbiology.
Figures


Similar articles
-
Shortening Buruli Ulcer Treatment with Combination Therapy Targeting the Respiratory Chain and Exploiting Mycobacterium ulcerans Gene Decay.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00426-19. doi: 10.1128/AAC.00426-19. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31036687 Free PMC article.
-
Impact of Dose, Duration, and Immune Status on Efficacy of Ultrashort Telacebec Regimens in Mouse Models of Buruli Ulcer.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0141821. doi: 10.1128/AAC.01418-21. Epub 2021 Aug 30. Antimicrob Agents Chemother. 2021. PMID: 34460302 Free PMC article.
-
Telacebec (Q203)-containing intermittent oral regimens sterilized mice infected with Mycobacterium ulcerans after only 16 doses.PLoS Negl Trop Dis. 2020 Aug 31;14(8):e0007857. doi: 10.1371/journal.pntd.0007857. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32866170 Free PMC article.
-
Buruli Ulcer: Review of a Neglected Skin Mycobacterial Disease.J Clin Microbiol. 2018 Mar 26;56(4):e01507-17. doi: 10.1128/JCM.01507-17. Print 2018 Apr. J Clin Microbiol. 2018. PMID: 29343539 Free PMC article. Review.
-
[Role of mycolactone in the nerve damage of Buruli ulcer (Mycobacterium ulcerans infection)].Nihon Hansenbyo Gakkai Zasshi. 2011 Feb;80(1):5-10. doi: 10.5025/hansen.80.5. Nihon Hansenbyo Gakkai Zasshi. 2011. PMID: 21404590 Review. Japanese.
Cited by
-
The Many Hosts of Mycobacteria 9 (MHM9): A conference report.Tuberculosis (Edinb). 2023 Sep;142:102377. doi: 10.1016/j.tube.2023.102377. Epub 2023 Jul 23. Tuberculosis (Edinb). 2023. PMID: 37531864 Free PMC article.
-
Targeting Mycobacterium tuberculosis iron-scavenging tools: a recent update on siderophores inhibitors.RSC Med Chem. 2023 Sep 6;14(10):1885-1913. doi: 10.1039/d3md00201b. eCollection 2023 Oct 18. RSC Med Chem. 2023. PMID: 37859726 Free PMC article. Review.
-
Structure of mycobacterial CIII2CIV2 respiratory supercomplex bound to the tuberculosis drug candidate telacebec (Q203).Elife. 2021 Sep 30;10:e71959. doi: 10.7554/eLife.71959. Elife. 2021. PMID: 34590581 Free PMC article.
-
Contribution of telacebec to novel drug regimens in a murine tuberculosis model.Antimicrob Agents Chemother. 2025 Jan 31;69(1):e0096224. doi: 10.1128/aac.00962-24. Epub 2024 Dec 9. Antimicrob Agents Chemother. 2025. PMID: 39651910 Free PMC article.
-
Mycobacterium Ulcerans Ulcer: Current Trends in Antimicrobial Management and Reconstructive Surgical Strategies.Life (Basel). 2025 Jul 13;15(7):1096. doi: 10.3390/life15071096. Life (Basel). 2025. PMID: 40724599 Free PMC article. Review.
References
-
- World Health Organization. 2017. Report from the meeting of the Buruli ulcer Technical Advisory Group. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

