Lubricant Investigation in Men to Inhibit Transmission of HPV Infection (LIMIT-HPV): design and methods for a randomised controlled trial
- PMID: 32205376
- PMCID: PMC7103806
- DOI: 10.1136/bmjopen-2019-035113
Lubricant Investigation in Men to Inhibit Transmission of HPV Infection (LIMIT-HPV): design and methods for a randomised controlled trial
Abstract
Introduction: Gay, bisexual and other men who have sex with men (gbMSM) have an increased risk of human papillomavirus (HPV) infection and HPV-associated diseases, such as anal cancer and anogenital warts. A carrageenan-based lubricant could prevent HPV infection, thereby reducing the disease burden in this population. This paper describes the protocol for the Lubricant Investigation in Men to Inhibit Transmission of HPV Infection (LIMIT-HPV) study, an ongoing randomised controlled trial (RCT), evaluating efficacy of a carrageenan-based personal lubricant in reducing type-specific anal HPV incidence and prevalence among sexually active gbMSM, efficacy by HIV status, safety and tolerability of the gel and participant adherence to the intervention.
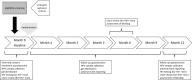
Methods and analysis: The study is a double-blinded, placebo-controlled RCT. Volunteer gbMSM 18 years and older are randomly assigned 1:1 to receive the treatment (a self-applied anal microbicide gel with carrageenan) or placebo (a self-applied placebo gel). At each visit, computerised questionnaires are used to collect data on sociodemographic and clinical variables, lifestyle, sexual behaviour and the gels' safety and tolerability. At baseline and each follow-up visit (months 1, 2, 3, 6, 9 and 12), nurses collect anal specimens tested for 36 HPV types (linear array assay). HIV status is determined at baseline and 12 months. The primary outcome is incidence of type-specific anal HPV infection(s) undetected at baseline. Secondary outcomes are prevalence of type-specific anal HPV infection, safety, tolerability and adherence. We aim to recruit 380 participants to attain the study's objectives. Data will be analysed using intention-to-treat and per-protocol approaches with subgroup analyses by HIV status.
Ethics and dissemination: Ethics approval was obtained by the Research Ethics Boards of McGill University, the McGill University Health Centre, Concordia University and Centre Hospitalier de l'Université de Montréal. Trial results will be disseminated through peer-reviewed publications and conference presentations.
Trial registration number: NCT02354144.
Keywords: HIV & AIDS; epidemiology; infection control; public health.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AdP’s clinic participates in pharmaceutical clinical trials for HIV antiretrovirals and HCV treatments (ViiV Healthcare, Janssen, Merck and Gilead), received honoraria for consulting on HIV antiretroviral regimen for ViiV Healthcare, and received grants from CIHR and FRQ-S outside the submitted work. EF reports grants and personal fees from Merck, grants, personal fees and non-financial support from Roche and personal fees from GSK, outside the submitted work. JT is a Merck employee. FC reports grants from Réseau FRQS-SIDA during the conduct of the study and grants to his institution for HPV-related work but outside of the submitted work from Merk Sharp and Dome, Roche Diagnostics and Becton Dickinson.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
