Clinicians' attitudes to oncology clinical practice guidelines and the barriers and facilitators to adherence: a mixed methods study protocol
- PMID: 32205377
- PMCID: PMC7103843
- DOI: 10.1136/bmjopen-2019-035448
Clinicians' attitudes to oncology clinical practice guidelines and the barriers and facilitators to adherence: a mixed methods study protocol
Abstract
Introduction: Clinical practice guidelines (CPGs) are designed to reduce inappropriate clinical variation and improve the quality of care. Barriers to CPGs include a lack of awareness of CPGs, access to them, time pressures and concerns regarding the evidence underpinning CPG development, implementation and dissemination. The objectives of this study are to assess clinicians' attitudes to CPGs for cancer treatment and the perceived barriers to and facilitators of CPG adherence in order to inform the implementation of cancer treatment CPGs.
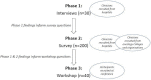
Methods and analysis: A mixed methods study will be conducted using a three-phase, sequential design, with each phase informing the next. In phase 1, a qualitative study using recorded interviews will investigate clinicians' attitudes to CPGs for cancer treatment and perceptions of barriers and facilitators to CPG adherence (n=30); interview transcripts will be analysed thematically. In phase 2, a survey will quantify the frequency of attitudes, barriers and facilitators identified in phase 1, in a broader clinical sample (n=200). In phase 3, a workshop forum will be held to facilitate discussions examining the implications of phase 1 and 2 findings for cancer CPG implementation strategies (n=40) leading to recommendations for improvements to practice. The workshop discussion will be recorded, and the transcript will be analysed thematically.
Ethics and dissemination: This study has received ethics approval in New South Wales, Australia (2019/ETH11722, #52019568810127). Study findings will be published in peer-reviewed journals and will form part of a doctoral thesis and be presented at national and international conferences.
Keywords: oncology; protocols & guidelines; quality in health care.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
References
-
- Australian Institute of Health and Welfare Cancer data in Australia. AIHW, 2018.
-
- Stewart BaW CP. International Agency for Research on Cancer : World cancer report 2014. Geneva: World Health Organisation, 2014.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources