Endorepellin evokes an angiostatic stress signaling cascade in endothelial cells
- PMID: 32205445
- PMCID: PMC7212646
- DOI: 10.1074/jbc.RA120.012525
Endorepellin evokes an angiostatic stress signaling cascade in endothelial cells
Abstract
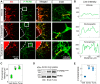
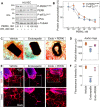
Endorepellin, the C-terminal fragment of the heparan sulfate proteoglycan perlecan, influences various signaling pathways in endothelial cells by binding to VEGFR2. In this study, we discovered that soluble endorepellin activates the canonical stress signaling pathway consisting of PERK, eIF2α, ATF4, and GADD45α. Specifically, endorepellin evoked transient activation of VEGFR2, which, in turn, phosphorylated PERK at Thr980 Subsequently, PERK phosphorylated eIF2α at Ser51, upregulating its downstream effector proteins ATF4 and GADD45α. RNAi-mediated knockdown of PERK or eIF2α abrogated the endorepellin-mediated up-regulation of GADD45α, the ultimate effector protein of this stress signaling cascade. To functionally validate these findings, we utilized an ex vivo model of angiogenesis. Exposure of the aortic rings embedded in 3D fibrillar collagen to recombinant endorepellin for 2-4 h activated PERK and induced GADD45α vis à vis vehicle-treated counterparts. Similar effects were obtained with the established cellular stress inducer tunicamycin. Notably, chronic exposure of aortic rings to endorepellin for 7-9 days markedly suppressed vessel sprouting, an angiostatic effect that was rescued by blocking PERK kinase activity. Our findings unravel a mechanism by which an extracellular matrix protein evokes stress signaling in endothelial cells, which leads to angiostasis.
Keywords: GADD45; PERK; angiogenesis; eIF2α; endothelial cell; proteoglycan; signal transduction; stress response; stress signaling.
© 2020 Kapoor et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

