An Algorithmic Immunohistochemical Approach to Define Tumor Type and Assign Site of Origin
- PMID: 32205473
- PMCID: PMC7700753
- DOI: 10.1097/PAP.0000000000000256
An Algorithmic Immunohistochemical Approach to Define Tumor Type and Assign Site of Origin
Abstract
Immunohistochemistry represents an indispensable complement to an epidemiology and morphology-driven approach to tumor diagnosis and site of origin assignment. This review reflects the state of my current practice, based on 15-years' experience in Pathology and a deep-dive into the literature, always striving to be better equipped to answer the age old questions, "What is it, and where is it from?" The tables and figures in this manuscript are the ones I "pull up on the computer" when I am teaching at the microscope and turn to myself when I am (frequently) stuck. This field is so exciting because I firmly believe that, through the application of next-generation immunohistochemistry, we can provide better answers than ever before. Specific topics covered in this review include (1) broad tumor classification and associated screening markers; (2) the role of cancer epidemiology in determining pretest probability; (3) broad-spectrum epithelial markers; (4) noncanonical expression of broad tumor class screening markers; (5) a morphologic pattern-based approach to poorly to undifferentiated malignant neoplasms; (6) a morphologic and immunohistochemical approach to define 4 main carcinoma types; (7) CK7/CK20 coordinate expression; (8) added value of semiquantitative immunohistochemical stain assessment; algorithmic immunohistochemical approaches to (9) "garden variety" adenocarcinomas presenting in the liver, (10) large polygonal cell adenocarcinomas, (11) the distinction of primary surface ovarian epithelial tumors with mucinous features from metastasis, (12) tumors presenting at alternative anatomic sites, (13) squamous cell carcinoma versus urothelial carcinoma, and neuroendocrine neoplasms, including (14) the distinction of pheochromocytoma/paraganglioma from well-differentiated neuroendocrine tumor, site of origin assignment in (15) well-differentiated neuroendocrine tumor and (16) poorly differentiated neuroendocrine carcinoma, and (17) the distinction of well-differentiated neuroendocrine tumor G3 from poorly differentiated neuroendocrine carcinoma; it concludes with (18) a discussion of diagnostic considerations in the broad-spectrum keratin/CD45/S-100-"triple-negative" neoplasm.
Figures





































































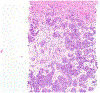


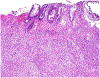



























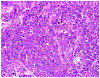






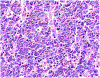











References
-
- McFarland M, Quick CM, McCluggage WG. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016;68(7):1013–20. - PubMed
-
- Pors J, Cheng A, Leo JM, Kinloch MA, Gilks B, Hoang L. A Comparison of GATA3, TTF1, CD10, and Calretinin in Identifying Mesonephric and Mesonephric-like Carcinomas of the Gynecologic Tract. The American journal of surgical pathology. 2018;42(12):1596–606. - PubMed
-
- Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133(19):3871–81. - PubMed
-
- Rohrer H Transcriptional control of differentiation and neurogenesis in autonomic ganglia. The European journal of neuroscience. 2011;34(10):1563–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

