Concurrent imaging of vascularization and metabolism in a mouse model of paraganglioma under anti-angiogenic treatment
- PMID: 32206105
- PMCID: PMC7069082
- DOI: 10.7150/thno.40687
Concurrent imaging of vascularization and metabolism in a mouse model of paraganglioma under anti-angiogenic treatment
Abstract
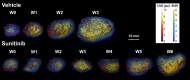
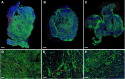
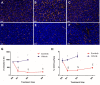
Rationale: Deregulation of metabolism and induction of vascularization are major hallmarks of cancer. Using a new multimodal preclinical imaging instrument, we explored a sequence of events leading to sunitinib-induced resistance in a murine model of paraganglioma (PGL) invalidated for the expression of succinate dehydrogenase subunit B (Sdhb-/-). Methods: Two groups of Sdhb-/- tumors bearing mice were treated with sunitinib (6 weeks) or vehicle (3 weeks). Concurrent Positron Emission Tomography (PET) with 2' -deoxy-2'-[18F]fluoro-D-glucose (FDG), Computed Tomography (CT) and Ultrafast Ultrasound Imaging (UUI) imaging sessions were performed once a week and ex vivo samples were analyzed by western blots and histology. Results: PET-CT-UUI enabled to detect a rapid growth of Sdhb-/- tumors with increased glycolysis and vascular development. Sunitinib treatment prevented tumor growth, vessel development and reduced FDG uptake at week 1 and 2 (W1-2). Thereafter, imaging revealed tumor escape from sunitinib treatment: FDG uptake in tumors increased at W3, followed by tumor growth and vessel development at W4-5. Perfused vessels were preferentially distributed in the hypermetabolic regions of the tumors and the perfused volume increased during escape from sunitinib treatment. Finally, initial changes in total lesion glycolysis and maximum vessel length at W1 were predictive of resistance to sunitinib. Conclusion: These results demonstrate an adaptive resistance of Sdhb-/- tumors to six weeks of sunitinib treatment. Early metabolic changes and delayed vessel architecture changes were detectable and predictable in vivo early during anti-angiogenic treatment. Simultaneous metabolic, anatomical and functional imaging can monitor precisely the effects of anti-angiogenic treatment of tumors.
Keywords: Cancer metabolism; SDHB; angiogenesis; multimodality imaging; paraganglioma; positron emission tomography; ultrafast-ultrasound imaging.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. 2016;388(10043):518–29. - PubMed
-
- Nassif E, Thibault C, Vano Y, Fournier L, Mauge L, Verkarre V. et al. Sunitinib in kidney cancer: 10 years of experience and development. Expert Rev Anticancer Ther. 2017;17(2):129–42. - PubMed
-
- Favier J, Amar L, Gimenez-Roqueplo A-P. Paraganglioma and phaeochromocytoma: from genetics to personalized medicine. Nat Rev Endocrinol. 2015;11(2):101–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

