Synthetic immunity by remote control
- PMID: 32206114
- PMCID: PMC7069089
- DOI: 10.7150/thno.41305
Synthetic immunity by remote control
Abstract
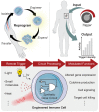
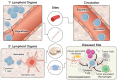
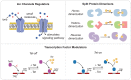
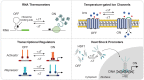
Cell-based immunotherapies, such as T cells engineered with chimeric antigen receptors (CARs), have the potential to cure patients of disease otherwise refractory to conventional treatments. Early-on-treatment and long-term durability of patient responses depend critically on the ability to control the potency of adoptively transferred T cells, as overactivation can lead to complications like cytokine release syndrome, and immunosuppression can result in ineffective responses to therapy. Drugs or biologics (e.g., cytokines) that modulate immune activity are limited by mass transport barriers that reduce the local effective drug concentration, and lack site or target cell specificity that results in toxicity. Emerging technologies that enable site-targeted, remote control of key T cell functions - including proliferation, antigen-sensing, and target-cell killing - have the potential to increase treatment precision and safety profile. These technologies are broadly applicable to other immune cells to expand immune cell therapies across many cancers and diseases. In this review, we highlight the opportunities, challenges and the current state-of-the-art for remote control of synthetic immunity.
Keywords: engineered cells; gene switches; immunotherapy; remote control; synthetic immunity.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Advances in Chimeric Antigen Receptor T-Cell Therapies for Solid Tumors.Clin Pharmacol Ther. 2019 Jan;105(1):71-78. doi: 10.1002/cpt.1280. Clin Pharmacol Ther. 2019. PMID: 30406956 Review.
-
Strategies for enhancing adoptive T-cell immunotherapy against solid tumors using engineered cytokine signaling and other modalities.Expert Opin Biol Ther. 2018 Jun;18(6):653-664. doi: 10.1080/14712598.2018.1473368. Epub 2018 May 14. Expert Opin Biol Ther. 2018. PMID: 29727246 Free PMC article. Review.
-
Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity.Br J Haematol. 2021 Apr;193(2):216-230. doi: 10.1111/bjh.17186. Epub 2020 Nov 20. Br J Haematol. 2021. PMID: 33216984 Free PMC article. Review.
-
Chimeric antigen receptor T-cell therapy beyond cancer: current practice and future prospects.Immunotherapy. 2020 Sep;12(13):1021-1034. doi: 10.2217/imt-2020-0009. Epub 2020 Jul 30. Immunotherapy. 2020. PMID: 32727249 Review.
-
Tuning CARs: recent advances in modulating chimeric antigen receptor (CAR) T cell activity for improved safety, efficacy, and flexibility.J Transl Med. 2023 Mar 15;21(1):197. doi: 10.1186/s12967-023-04041-6. J Transl Med. 2023. PMID: 36922828 Free PMC article. Review.
Cited by
-
Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound.Adv Drug Deliv Rev. 2022 Jan;180:114043. doi: 10.1016/j.addr.2021.114043. Epub 2021 Nov 18. Adv Drug Deliv Rev. 2022. PMID: 34801617 Free PMC article. Review.
-
Targeting phosphatidylserine for Cancer therapy: prospects and challenges.Theranostics. 2020 Jul 23;10(20):9214-9229. doi: 10.7150/thno.45125. eCollection 2020. Theranostics. 2020. PMID: 32802188 Free PMC article. Review.
-
Remote controlling of CAR-T cells and toxicity management: Molecular switches and next generation CARs.Transl Oncol. 2021 Jun;14(6):101070. doi: 10.1016/j.tranon.2021.101070. Epub 2021 Mar 28. Transl Oncol. 2021. PMID: 33789222 Free PMC article. Review.
-
Enhanced intratumoural activity of CAR T cells engineered to produce immunomodulators under photothermal control.Nat Biomed Eng. 2021 Nov;5(11):1348-1359. doi: 10.1038/s41551-021-00781-2. Epub 2021 Aug 12. Nat Biomed Eng. 2021. PMID: 34385695 Free PMC article.
-
Magnetic systems for cancer immunotherapy.Acta Pharm Sin B. 2021 Aug;11(8):2172-2196. doi: 10.1016/j.apsb.2021.03.023. Epub 2021 Apr 30. Acta Pharm Sin B. 2021. PMID: 34522583 Free PMC article. Review.
References
-
- Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37:1425–1434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

