Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics
- PMID: 32206116
- PMCID: PMC7069071
- DOI: 10.7150/thno.41580
Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics
Abstract
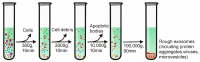
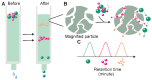
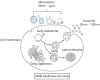
Exosomes are small extracellular vesicles with diameters of 30-150 nm. In both physiological and pathological conditions, nearly all types of cells can release exosomes, which play important roles in cell communication and epigenetic regulation by transporting crucial protein and genetic materials such as miRNA, mRNA, and DNA. Consequently, exosome-based disease diagnosis and therapeutic methods have been intensively investigated. However, as in any natural science field, the in-depth investigation of exosomes relies heavily on technological advances. Historically, the two main technical hindrances that have restricted the basic and applied researches of exosomes include, first, how to simplify the extraction and improve the yield of exosomes and, second, how to effectively distinguish exosomes from other extracellular vesicles, especially functional microvesicles. Over the past few decades, although a standardized exosome isolation method has still not become available, a number of techniques have been established through exploration of the biochemical and physicochemical features of exosomes. In this work, by comprehensively analyzing the progresses in exosome separation strategies, we provide a panoramic view of current exosome isolation techniques, providing perspectives toward the development of novel approaches for high-efficient exosome isolation from various types of biological matrices. In addition, from the perspective of exosome-based diagnosis and therapeutics, we emphasize the issue of quantitative exosome and microvesicle separation.
Keywords: Exosome; diagnosis; extracellular vesicle; microfluidic; microvesicle; separation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures











References
-
- Pickett J. Cell signalling - New communication skills. Nat Rev Mol Cell Bio. 2007;8:423. -
-
- Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A. et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36:328–34. - PubMed
-
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle Formation during Reticulocyte Maturation - Association of Plasma-Membrane Activities with Released Vesicles (Exosomes) J Biol Chem. 1987;262:9412–20. - PubMed
-
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–U72. - PubMed
-
- Kilchert C, Wittmann S, Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat Rev Mol Cell Bio. 2016;17:227–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

