A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer
- PMID: 32206117
- PMCID: PMC7069095
- DOI: 10.7150/thno.41677
A cell-permeable peptide-based PROTAC against the oncoprotein CREPT proficiently inhibits pancreatic cancer
Abstract
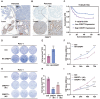
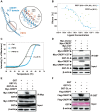
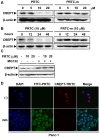
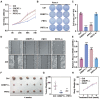
Cancers remain a threat to human health due to the lack of effective therapeutic strategies. Great effort has been devoted to the discovery of drug targets to treat cancers, but novel oncoproteins still need to be unveiled for efficient therapy. Methods: We show that CREPT is highly expressed in pancreatic cancer and is associated with poor disease-free survival. CREPT overexpression promotes but CREPT deletion blocks colony formation and proliferation of pancreatic cancer cells. To provide a proof of concept for CREPT as a new target for the inhibition of pancreatic cancer, we designed a cell-permeable peptide-based proteolysis targeting chimera (PROTAC), named PRTC, based on the homodimerized leucine-zipper-like motif in the C-terminus domain of CREPT to induce its degradation in vivo. Results: PRTC has high affinity for CREPT, with Kd = 0.34 +/- 0.11 μM and is able to permeate into cells because of the attached membrane-transportable peptide RRRRK. PRTC effectively induces CREPT degradation in a proteasome-dependent manner. Intriguingly, PRTC inhibits colony formation, cell proliferation, and motility in pancreatic cancer cells and ultimately impairs xenograft tumor growth, comparable to the effect of CREPT deletion. Conclusions: PRTC-induced degradation of CREPT leads to inhibition of tumor growth, which is promising for the development of new drugs against pancreatic cancer. In addition, using an interacting motif based on the dimerized structure of proteins may be a new way to design a PROTAC aiming at degrading any protein without known interacting small molecules or peptides.
Keywords: CREPT; PROTAC; degradation; drug target; pancreatic cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
CREPT regulated by miR-138 promotes breast cancer progression.Biochem Biophys Res Commun. 2017 Nov 4;493(1):263-269. doi: 10.1016/j.bbrc.2017.09.033. Epub 2017 Sep 8. Biochem Biophys Res Commun. 2017. PMID: 28893536
-
Inhibition of CREPT restrains gastric cancer growth by regulation of cycle arrest, migration and apoptosis via ROS-regulated p53 pathway.Biochem Biophys Res Commun. 2018 Feb 19;496(4):1183-1190. doi: 10.1016/j.bbrc.2018.01.167. Epub 2018 Jan 31. Biochem Biophys Res Commun. 2018. PMID: 29402413
-
Cell cycle-related and expression-elevated protein in tumor overexpression is associated with proliferation behaviors and poor prognosis in non-small-cell lung cancer.Cancer Sci. 2018 Apr;109(4):1012-1023. doi: 10.1111/cas.13524. Epub 2018 Mar 5. Cancer Sci. 2018. PMID: 29397041 Free PMC article.
-
Current understanding of CREPT and p15RS, carboxy-terminal domain (CTD)-interacting proteins, in human cancers.Oncogene. 2021 Jan;40(4):705-716. doi: 10.1038/s41388-020-01544-0. Epub 2020 Nov 25. Oncogene. 2021. PMID: 33239754 Review.
-
Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy.J Hematol Oncol. 2020 May 13;13(1):50. doi: 10.1186/s13045-020-00885-3. J Hematol Oncol. 2020. PMID: 32404196 Free PMC article. Review.
Cited by
-
CREPT is required for murine stem cell maintenance during intestinal regeneration.Nat Commun. 2021 Jan 11;12(1):270. doi: 10.1038/s41467-020-20636-9. Nat Commun. 2021. PMID: 33431892 Free PMC article.
-
PROTACs in gastrointestinal cancers.Mol Ther Oncolytics. 2022 Nov 3;27:204-223. doi: 10.1016/j.omto.2022.10.012. eCollection 2022 Dec 15. Mol Ther Oncolytics. 2022. PMID: 36420306 Free PMC article. Review.
-
Microscale Thermophoresis as a Tool to Study Protein Interactions and Their Implication in Human Diseases.Int J Mol Sci. 2022 Jul 12;23(14):7672. doi: 10.3390/ijms23147672. Int J Mol Sci. 2022. PMID: 35887019 Free PMC article. Review.
-
Development of DNA Aptamer-Based PROTACs That Degrade the Estrogen Receptor.ACS Med Chem Lett. 2023 May 19;14(6):827-832. doi: 10.1021/acsmedchemlett.3c00126. eCollection 2023 Jun 8. ACS Med Chem Lett. 2023. PMID: 37312841 Free PMC article.
-
Advancing Design Strategy of PROTACs for Cancer Therapy.MedComm (2020). 2025 Jun 25;6(7):e70258. doi: 10.1002/mco2.70258. eCollection 2025 Jul. MedComm (2020). 2025. PMID: 40567248 Free PMC article. Review.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. - PubMed
-
- Fidler MM, Bray F, Soerjomataram I. The global cancer burden and human development: A review. Scand J Public Health. 2018;46:27–36. - PubMed
-
- Beaver CC, Magnan MA. Managing Chemotherapy Side Effects: Achieving Reliable and Equitable Outcomes. Clin J Oncol Nurs. 2016;20:589–91. - PubMed
-
- Mavrogenis AF, Megaloikonomos PD, Panagopoulos GN, Papagelopoulos PJ, Theophanides T, Anastassopoulou J. Side Effects of Radiation in Bone and Cartilage: An FT-IR Analysis. J Long Term Eff Med Implants. 2015;25:289–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

