Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy
- PMID: 32206118
- PMCID: PMC7069070
- DOI: 10.7150/thno.42008
Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy
Abstract
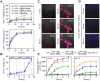
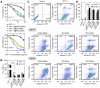
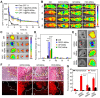
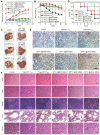
Rationale: Antitumor drug delivery faces multiple barriers that require consecutively achieving tumor targeting, selective cellular uptake and sufficient intracellular drug dosage. Methods: Herein, we designed smart nanoparticles (GPDC-MSNs) that can accumulate stepwise in tumor tissues, selectively enter cancer cells by responding to the acidic tumor extracellular environment, and achieve considerable drug release in the intracellular microenvironment. The GPDC-MSNs comprise the synthesized material galactosyl-conjugated PEO-PPO-PEO (Gal-P123) for hepatocellular carcinoma (HCC) targeting, the tumor extracellular pH-responsive lipid (2E)-4-(dioleostearin)-amino-4-carbonyl-2-butenonic (DC) for selective cellular internalization, and antitumor drug irinotecan (CPT-11)-loaded mesoporous silica nanoparticles (MSNs) for on-demand intracellular drug release. Results: GPDC-MSNs are negatively charged at pH 7.4 and promote active HCC targeting mediated by the asialoglycoprotein receptor. Upon reaching the weakly acidic tumor microenvironment, the nanoparticles undergo charge conversion to neutral, enhancing cellular internalization. Moreover, the encapsulated CPT-11 can be retained within GPDC-MSNs in the blood circulation but undergo intracellular burst release, which facilitates the apoptosis of tumor cells. GPDC-MSNs significantly increased HCC selectivity in vivo and exhibited up to 25 times higher accumulation in tumor tissue than in normal hepatic tissue, thus achieving superior antitumor efficacy and low systemic toxicity. Conclusion: This stepwise-responsive nanoparticle should serve as a valuable platform and promising strategy for HCC treatment.
Keywords: charge conversion; drug delivery; hepatocellular carcinoma; pH sensitive; tumor targeting.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
[Antitumor efficacy of irinotecan-loaded galactosyl modified lipid bilayer-coated mesoporous silica nanoparticles against hepatocellular carcinoma cells].Yao Xue Xue Bao. 2014 May;49(5):718-25. Yao Xue Xue Bao. 2014. PMID: 25151746 Chinese.
-
Biofunctionalized polymer-lipid supported mesoporous silica nanoparticles for release of chemotherapeutics in multidrug resistant cancer cells.Biomaterials. 2014 Apr;35(11):3650-65. doi: 10.1016/j.biomaterials.2014.01.013. Epub 2014 Jan 24. Biomaterials. 2014. PMID: 24462359
-
Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence.Acta Biomater. 2019 Dec;100:352-364. doi: 10.1016/j.actbio.2019.09.034. Epub 2019 Sep 26. Acta Biomater. 2019. PMID: 31563690
-
Recent advances in mesoporous silica nanoparticles for antitumor therapy: our contribution.Biomater Sci. 2016 May 26;4(5):803-13. doi: 10.1039/c6bm00039h. Epub 2016 Feb 23. Biomater Sci. 2016. PMID: 26902682 Review.
-
Recent Advances in Asialoglycoprotein Receptor and Glycyrrhetinic Acid Receptor-Mediated and/or pH-Responsive Hepatocellular Carcinoma- Targeted Drug Delivery.Curr Med Chem. 2021;28(8):1508-1534. doi: 10.2174/0929867327666200505085756. Curr Med Chem. 2021. PMID: 32368967 Review.
Cited by
-
Smart drug delivery systems for precise cancer therapy.Acta Pharm Sin B. 2022 Nov;12(11):4098-4121. doi: 10.1016/j.apsb.2022.08.013. Epub 2022 Aug 22. Acta Pharm Sin B. 2022. PMID: 36386470 Free PMC article. Review.
-
Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma.J Exp Clin Cancer Res. 2024 Aug 30;43(1):247. doi: 10.1186/s13046-024-03167-9. J Exp Clin Cancer Res. 2024. PMID: 39215325 Free PMC article.
-
Drug delivery systems based on mesoporous silica nanoparticles for the management of hepatic diseases.Acta Pharm Sin B. 2025 Feb;15(2):809-833. doi: 10.1016/j.apsb.2024.12.015. Epub 2024 Dec 20. Acta Pharm Sin B. 2025. PMID: 40177563 Free PMC article. Review.
-
Mushroom-derived bioactive components with definite structures in alleviating the pathogenesis of Alzheimer's disease.Front Pharmacol. 2024 May 21;15:1373660. doi: 10.3389/fphar.2024.1373660. eCollection 2024. Front Pharmacol. 2024. PMID: 38835656 Free PMC article. Review.
-
Nanomaterials for liver cancer targeting: research progress and future prospects.Front Immunol. 2025 Feb 28;16:1496498. doi: 10.3389/fimmu.2025.1496498. eCollection 2025. Front Immunol. 2025. PMID: 40092984 Free PMC article. Review.
References
-
- Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65. - PubMed
-
- Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15:333–48. - PubMed
-
- Sun Q, Zhou Z, Qiu N, Shen Y. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29:1606628–45. - PubMed
-
- Cheng R, Meng FH, Deng C, Zhong ZY. Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy. Nano Today. 2015;10:656–70.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

