Flow induced particle separation and collection through linear array pillar microfluidics device
- PMID: 32206158
- PMCID: PMC7082176
- DOI: 10.1063/1.5143656
Flow induced particle separation and collection through linear array pillar microfluidics device
Abstract
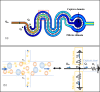
Particle filtration and concentration have great significance in a multitude of applications. Physical filters are nearly indispensable in conventional separation processes. Similarly, microfabrication-based physical filters are gaining popularity as size-based particle sorters, separators, and prefiltration structures for microfluidics platforms. The work presented here introduces a linear combination of obstructions to provide size contrast-based particle separation. Polystyrene particles that are captured along the crossflow filters are packed in the direction of the dead-end filters. Separation of polydisperse suspension of 5 μm and 10 μm diameter polystyrene microspheres is attained with capture efficiency for larger particles as 95%. Blood suspension is used for biocharacterization of the device. A flow induced method is used to improve particle capture uniformity in a single microchannel and reduce microgap clogging to about 30%. This concept is extended to obtain semiquantification obtained by comparison of the initial particle concentration to captured-particle occupancy in a microfiltration channel.
Copyright © 2020 Author(s).
Figures












References
LinkOut - more resources
Full Text Sources
