Circulating cytokines and outcome in metastatic colorectal cancer patients treated with regorafenib
- PMID: 32206180
- PMCID: PMC7081116
- DOI: 10.4251/wjgo.v12.i3.301
Circulating cytokines and outcome in metastatic colorectal cancer patients treated with regorafenib
Abstract
Background: Regorafenib is an oral small-molecule multikinase inhibitor approved in third or later line of treatment for patients with metastatic colorectal cancer (mCRC). Regorafenib has shown significant benefits in overall survival and progression free survival in two phase III trials compared to placebo in patients with mCRC who had progressed on previous therapy.
Aim: To identify an immune profile that might specifically correlate with the outcome in patients treated with regorafenib.
Methods: Blood samples were collected from 17 patients before treatment with regorafenib and from 6 healthy volunteers. The proteins evaluated (TNF-α, TGF-β, VEGF, CCL-2, CCL-4, and CCL-5) were selected on the basis of their roles in angiogenesis and colorectal cancer pathogenesis.
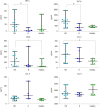
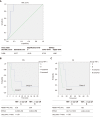
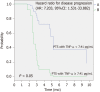
Results: We found that TNF-α basal level was significantly higher in mCRC patients compared to healthy individuals. Non Responder (NR) patients showing progression of disease (n = 12) had higher basal level of TGF-β, TNF-α, VEGF, CCL-2 and CCL-5 compared to Responder (R) patients (complete response CR, n = 1; partial response PR, n = 1; Stable Disease SD, n = 3). On the contrary, plasma basal level of CCL-4 was higher in R compared to NR patients. High values of TGF-β and TNF-α negatively correlated with progression free survival.
Conclusion: These results suggest a cytokine signature potentially able to discriminate between R and NR patients to treatment with regorafenib.
Keywords: Angiogenesis; Colorectal cancer; Cytokines; Multikinase inhibitor.
©The Author(s) 2019. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Figures

References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. - PubMed
-
- Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, Eguchi Nakajima T, Baba E, Tsuda M, Moriwaki T, Esaki T, Tsuji Y, Muro K, Taira K, Denda T, Funai S, Shinozaki K, Yamashita H, Sugimoto N, Okuno T, Nishina T, Umeki M, Kurimoto T, Takayama T, Tsuji A, Yoshida M, Hosokawa A, Shibata Y, Suyama K, Okabe M, Suzuki K, Seki N, Kawakami K, Sato M, Fujikawa K, Hirashima T, Shimura T, Taku K, Otsuji T, Tamura F, Shinozaki E, Nakashima K, Hara H, Tsushima T, Ando M, Morita S, Boku N, Hyodo I. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G) Ann Oncol. 2016;27:1539–1546. - PubMed
-
- Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–255. - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

