Wavelength-gated photoreversible polymerization and topology control
- PMID: 32206267
- PMCID: PMC7069517
- DOI: 10.1039/c9sc05381f
Wavelength-gated photoreversible polymerization and topology control
Abstract
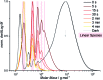
We exploit the wavelength dependence of [2 + 2] photocycloadditions and -reversions of styrylpyrene to exert unprecedented control over the photoreversible polymerization and topology of telechelic building blocks. Blue light (λ max = 460 nm) initiates a catalyst-free polymerization yielding high molar mass polymers (M n = 60 000 g mol-1), which are stable at wavelengths exceeding 430 nm, yet highly responsive to shorter wavelengths. UVB irradiation (λ max = 330 nm) induces a rapid depolymerization affording linear oligomers, whereas violet light (λ max = 410 nm) generates cyclic entities. Thus, different colors of light allow switching between a depolymerization that either proceeds through cyclic or linear topologies. The light-controlled topology formation was evidenced by correlation of mass spectrometry (MS) with size exclusion chromatography (SEC) and ion mobility data. Critically, the color-guided topology control was also possible with ambient laboratory light affording cyclic oligomers, while sunlight activated the linear depolymerization pathway. These findings suggest that light not only induces polymerization and depolymerization but that its color can control the topological outcomes.
This journal is © The Royal Society of Chemistry 2020.
Figures




References
LinkOut - more resources
Full Text Sources

