In vivo assessment of functional and morphological alterations in tumors under treatment using OCT-angiography combined with OCT-elastography
- PMID: 32206416
- PMCID: PMC7075625
- DOI: 10.1364/BOE.386419
In vivo assessment of functional and morphological alterations in tumors under treatment using OCT-angiography combined with OCT-elastography
Abstract
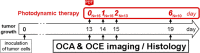
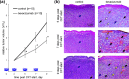
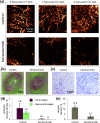
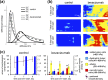
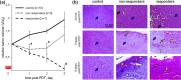
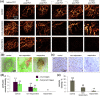
Emerging methods of anti-tumor therapies require new approaches to tumor response evaluation, especially enabling label-free diagnostics and in vivo utilization. Here, to assess the tumor early reaction and predict its long-term response, for the first time we apply in combination the recently developed OCT extensions - optical coherence angiography (OCA) and compressional optical coherence elastography (OCE), thus enabling complementary functional/microstructural tumor characterization. We study two vascular-targeted therapies of different types, (1) anti-angiogenic chemotherapy (ChT) and (2) photodynamic therapy (PDT), aimed to indirectly kill tumor cells through blood supply injury. Despite different mechanisms of anti-angiogenic action for ChT and PDT, in both cases OCA demonstrated high sensitivity to blood perfusion cessation. The new method of OCE-based morphological segmentation revealed very similar histological structure alterations. The OCE results showed high correlation with conventional histology in evaluating percentages of necrotic and viable tumor zones. Such possibilities make OCE an attractive tool enabling previously inaccessible in vivo monitoring of individual tumor response to therapies without taking multiple biopsies.
© 2020 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures




References
-
- Eisenhauer E. A., Therasse P., Bogaerts J., Schwartz L. H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., Rubinstein L., Shankar L., Dodd L., Kaplan R., Lacombe D., Verweij J., “New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1),” Eur. J. Cancer 45(2), 228–247 (2009).10.1016/j.ejca.2008.10.026 - DOI - PubMed
-
- Demidov V., Matveev L. A., Demidova O., Matveyev A. L., Zaitsev V. Y., Flueraru C., Vitkin I. A., “Analysis of low-scattering regions in optical coherence tomography: applications to neurography and lymphangiography,” Biomed. Opt. Express 10(8), 4207–4219 (2019).10.1364/BOE.10.004207 - DOI - PMC - PubMed
-
- Standish B. A., Lee K. K., Jin X., Mariampillai A., Munce N. R., Wood M. F., Wilson B. C., Vitkin I. A., Yang V. X., “Interstitial Doppler optical coherence tomography as a local tumor necrosis predictor in photodynamic therapy of prostatic carcinoma: an in vivo study,” Cancer Res. 68(23), 9987–9995 (2008).10.1158/0008-5472.CAN-08-1128 - DOI - PubMed
-
- Sirotkina M. A., Matveev L. A., Shirmanova M. V., Zaitsev V. Y., Buyanova N. L., Elagin V. V., Gelikonov G. V., Kuznetsov S. S., Kiseleva E. B., Moiseev A. A., Gamayunov S. V., Zagaynova E. V., Feldchtein F. I., Vitkin A., Gladkova N. D., “Photodynamic therapy monitoring with optical coherence angiography,” Sci. Rep. 7(1), 41506 (2017).10.1038/srep41506 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical
