Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial
- PMID: 32206483
- PMCID: PMC7082467
- DOI: 10.1007/s13340-019-00412-x
Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA® trial
Abstract
Objective: Linagliptin, a dipeptidyl peptidase-4 inhibitor, demonstrated cardiovascular and renal safety in type 2 diabetes mellitus (T2DM) patients with established cardiovascular disease (CVD) with albuminuria and/or kidney disease in the multinational CARMELINA® trial. We investigated the effects of linagliptin in Asian patients in CARMELINA®.
Methods: T2DM patients with HbA1c 6.5-10.0% and established CVD with urinary albumin-to-creatinine ratio (UACR) > 30 mg/g, and/or prevalent kidney disease (estimated glomerular filtration rate [eGFR] 15-< 45 ml/min/1.73 m2 or ≥ 45-75 with UACR > 200 mg/g), were randomized to linagliptin or placebo added to usual care. The primary endpoint was time to first occurrence of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke (3-point MACE).
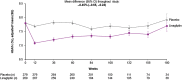
Results: Of the 6979 patients, 555 (8.0%) were Asians living in Asia. During a median follow-up of 2.2 years, 3-point MACE occurred in 29/272 (10.7%) and 33/283 (11.7%) of linagliptin and placebo patients, respectively (hazard ratio [HR] 0.90; 95% confidence interval [CI] 0.55-1.48), consistent with the overall population (HR 1.02; 95% CI 0.89-1.17; P value for treatment-by-region interaction: 0.3349). Similar neutrality in Asian patients was seen for other cardiorenal events including the secondary kidney endpoint of death from renal failure, progression to end-stage kidney disease, or ≥ 40% eGFR decrease (HR 0.96; 95% CI 0.58-1.59). Linagliptin was associated with a nominal decrease in the risk of hospitalization for heart failure (HR 0.47; 95% CI 0.24-0.95). Overall in Asian patients, linagliptin had an adverse event rate similar to placebo, consistent with the overall population.
Conclusions: Linagliptin showed cardiovascular and renal safety in Asian patients with T2DM and established CVD with albuminuria and/or kidney disease.
Keywords: Cardiovascular diseases; Diabetes mellitus, type 2; Prescription drugs; Renal insufficiency, chronic.
© The Japan Diabetes Society 2019.
Conflict of interest statement
Conflicts of interestN.I. has received honoraria from Kowa company; research funding from Mitsubishi Tanabe, Daiichi Sankyo and AstraZeneca; and subsidies/donations from Takeda, MSD, Ono, Sanofi, Japan Tobacco Inc., Mitsubishi Tanabe, Novartis, Boehringer Ingelheim, Kyowa Kirin, Astellas, Daiichi Sankyo, Kissei Pharmaceutical, Dainippon Pharma, Sanwa kagaku, Eli Lilly, Novo Nordisk, Teijin Pharma, and Taisho-Toyama Pharma. W.Y. has attended advisory boards for Novo Nordisk; received investigator-initiated trial research funds from AstraZeneca; been a speaker for Novo Nordisk, Bayer, Sanofi Aventis, Merck Sharp & Dohme China, AstraZeneca, Eli Lilly, Boehringer Ingelheim, and Servier; and received honorarium and travel support as an advisory board member from Merck & Co., Inc. H.W. has received honoraria from Eli Lilly, Mitsubishi Tanabe Pharma, Sanofi, Takeda Pharmaceutical Company, Novartis Pharma, Nippon Boehringer Ingelheim, Daiichi Sankyo, Ono Pharmaceutical, Astellas Pharma, FUJIFILM Pharma, Terumo Corporation, MSD; research funding from Eli Lilly, Novartis Pharma, Sanwa kagaku; subsidies/donations from Mitsubishi Tanabe Pharma, Kissei Pharmaceutical, Nippon Boehringer Ingelheim, Novartis Pharma, Sumitomo Dainippon Pharma, Sanofi, MSD, Pfizer Japan, Astellas Pharma, Takeda Pharmaceutical Company, Novo Nordisk, Teijin Pharma; and departmental endowments from Takeda Pharmaceutical Company, MSD, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Kowa company, and Sanwa kagaku. L.J. has received consulting and lecture fees from Eli Lilly and Company, Bristol-Myers Squibb, Novartis, Novo Nordisk, Merck, Bayer, MSD, Takeda, Sanofi, Roche, Boehringer Ingelheim, and AstraZeneca; and research support from Roche, Sanofi, MSD, AstraZeneca, Novartis, and Bristol-Myers Squibb. S.S., E.P., T.O., O.E.J., J.T.G., and M.v.E are employees of Boehringer Ingelheim. J.R. has served on scientific advisory boards and received honoraria or consulting fees from Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Boehringer Ingelheim, and Intarcia; he has also received grants/research support from Merck, Pfizer, Sanofi, Novo Nordisk, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Genentech, Janssen, Lexicon, Boehringer Ingelheim, and Intarcia. V.P. has received research support from the Australian National Health and Medical Research Council (Project and Program Grant); served on steering committees for trials supported by AbbVie, Boehringer Ingelheim, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Novo Nordisk, Pfizer, Retrophin, and Tricida; and served on advisory boards, spoken at scientific meetings, or both for AbbVie, Astellas Pharma, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect Corporation, Eli Lilly, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Roche, Sanofi, Servier, and Vitae. He has a policy of having honoraria paid to his employer. C.W. has received fees for advisory services to Boehringer Ingelheim and MSD as well as honoraria for lecturing from AstraZeneca, Eli Lilly and Sanofi. M.E.C. has received fees for advisory services and honoraria from Boehringer Ingelheim, Sanofi, Servier, Bayer, Astra Zeneca, Reata, MundiPharma and MSD and a grant from NovoNordisk. J.H.A. has received personal fees from Abbvie, Bristol-Myers Squibb, CSL Behring, Janssen Pharmaceutics, Novo Nordisk, Pfizer, Portola, and Teikoku; and institutional research support from Boehringer Ingelheim, Bristol-Myers Squibb, Cryolife, CSL Behring, Tenax Therapeutics, and VoluMetrix. I.K. has received honororia from Astellas Pharma Inc., MSD K.K., Edwards Laboratories Corporation, Otsuka Pharmaceutical Co. Ltd, Kowa Company, Ltd., Daiichi Sankyo Company, Limited, Taisho Pharma Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma Limited, Toa Eiyo Ltd, Bayer Yakuhin, Ltd, Terumo Corporation, Nipro Corporation; research funding from Ono Pharmaceutical Co., Ltd; and subsidies/donations from Actelion Pharmaceuticals Japan Ltd. Astellas Pharma Inc., Amgen Astellas BioPharma K.K., AstraZeneca K.K., MSD K.K., Shionogi & Co., Ltd, Daiichi Sankyo Company, Limited, Takeda Pharmacentical Company Limited, Toa Eiyo Ltd, Nippon Boehringer Ingelheim Co., Ltd, Bayer Yakuhin, Ltd, and Pfizer Japan Inc. M.N. is an advisor for KHK, Astellas, GSK, Daiichi-Sankyo, Mitsubishi-Tanabe, JT, Boehringer-Ingelheim; has received honoraria from KHK, Astellas, AstraZeneca, GSK, Daiichi-Sankyo, Mitubishi-Tanabe, Chugai, Torii, JT; manuscript fees from KHK; research funding from JT, KHK; and subsidies/donations from KHK, Astellas, Ono, Daiichi-Sankyo, Takeda, Mitsubishi-Tanabe, Chugai, Torii, MSD, Otsuka, Dainippon-Sumitomo, JT, and Boehringer Ingelheim.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
