Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies
- PMID: 32206624
- PMCID: PMC7073369
- DOI: 10.5223/pghn.2020.23.2.121
Long-Term Efficacy of Anti-Tumor Necrosis Factor Agents in Pediatric Luminal Crohn's Disease: A Systematic Review of Real-World Evidence Studies
Abstract
Purpose: To determine the long-term efficacy of the anti-tumor necrosis factor (TNF) agents, infliximab (IFX) and adalimumab (ADA), in pediatric luminal Crohn's disease (CD) by performing a systematic literature review.
Methods: An electronic search was performed in Medline, Embase, and the Cochrane Library from inception to September 26, 2019. Eligible studies were cohort studies with observation periods that exceeded 1 year. Studies that reported time-to-event analyses were included. Events were defined as discontinuation of anti-TNF therapy for secondary loss of response. We extracted the probabilities of continuing anti-TNF therapy 1, 2, and 3 years after initiation.
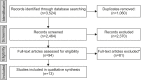
Results: In total, 2,464 papers were screened, 94 were selected for full text review, and 13 studies (11 on IFX, 2 on ADA) met our eligibility criteria for inclusion. After 1 year, 83-97% of patients were still receiving IFX therapy. After 2 and 3 years the probability of continuing IFX therapy decreased to 67-91% and 61-85%, respectively. In total, 5 of the 11 studies subgrouped by concomitant medication consistently showed that the probabilities of continuing IFX therapy in patients with prolonged immunomodulator use were higher than those in patients on IFX monotherapy.
Conclusion: This review of real-world evidence studies confirms the long-term therapeutic benefit of IFX therapy in diverse cohorts of children with luminal CD. Moreover, it supports the view that combination therapy with an immunomodulator prolongs the durability of IFX therapy in patients who previously failed to recover following first-line therapy. The limited number of time-to-event studies in patients on ADA prevented us from drawing definite conclusions about its long-term efficacy.
Keywords: Adalimumab; Crohn disease; Infliximab; Pediatrics; Survival analysis; Systematic review; Treatment outcome.
Copyright © 2020 by The Korean Society of Pediatric Gastroenterology, Hepatology and Nutrition.
Conflict of interest statement
Conflict of Interest: P.F. van Rheenen received speaker's fees from Janssen Pharmaceuticals and Abbvie.
Figures

References
-
- Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, et al. European Crohn's and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014;8:1179–1207. - PubMed
-
- Mack DR, Benchimol EI, Critch J, deBruyn J, Tse F, Moayyedi P, et al. Canadian Association of Gastroenterology clinical practice guideline for the medical management of pediatric luminal Crohn's disease. Gastroenterology. 2019;157:320–348. - PubMed
-
- Hyams J, Crandall W, Kugathasan S, Griffiths A, Olson A, Johanns J, et al. REACH Study Group. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132:863–873. quiz 1165-6. - PubMed
-
- Hyams JS, Griffiths A, Markowitz J, Baldassano RN, Faubion WA, Jr, Colletti RB, et al. Safety and efficacy of adalimumab for moderate to severe Crohn's disease in children. Gastroenterology. 2012;143:365–74.e2. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous