Increased mTOR activation in idiopathic multicentric Castleman disease
- PMID: 32206779
- PMCID: PMC7205815
- DOI: 10.1182/blood.2019002792
Increased mTOR activation in idiopathic multicentric Castleman disease
Abstract
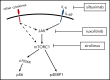
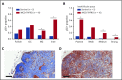
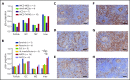
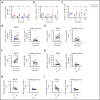
Idiopathic multicentric Castleman disease (iMCD) is a rare and poorly understood hematologic disorder characterized by lymphadenopathy, systemic inflammation, cytopenias, and life-threatening multiorgan dysfunction. Interleukin-6 (IL-6) inhibition effectively treats approximately one-third of patients. Limited options exist for nonresponders, because the etiology, dysregulated cell types, and signaling pathways are unknown. We previously reported 3 anti-IL-6 nonresponders with increased mTOR activation who responded to mTOR inhibition with sirolimus. We investigated mTOR signaling in tissue and serum proteomes from iMCD patients and controls. mTOR activation was increased in the interfollicular space of iMCD lymph nodes (N = 26) compared with control lymph nodes by immunohistochemistry (IHC) for pS6, p4EBP1, and p70S6K, known effectors and readouts of mTORC1 activation. IHC for pS6 also revealed increased mTOR activation in iMCD compared with Hodgkin lymphoma, systemic lupus erythematosus, and reactive lymph nodes, suggesting that the mTOR activation in iMCD is not just a product of lymphoproliferation/inflammatory lymphadenopathy. Further, the degree of mTOR activation in iMCD was comparable to autoimmune lymphoproliferative syndrome, a disease driven by mTOR hyperactivation that responds to sirolimus treatment. Gene set enrichment analysis of serum proteomic data from iMCD patients (n = 88) and controls (n = 42) showed significantly enriched mTORC1 signaling. Finally, functional studies revealed increased baseline mTOR pathway activation in peripheral monocytes and T cells from iMCD remission samples compared with healthy controls. IL-6 stimulation augmented mTOR activation in iMCD patients, which was abrogated with JAK1/2 inhibition. These findings support mTOR activation as a novel therapeutic target for iMCD, which is being investigated through a trial of sirolimus (NCT03933904).
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: D.C.F. receives research funding from EUSA Pharma for the ACCELERATE registry (NCT02817997, formerly funded by Janssen Pharmaceuticals), and Pfizer provides study drug with no associated research funding for the clinical trial of sirolimus (NCT03933904). D.T.T. sits on an advisory board for Janssen Pharmaceuticals. There is a pending provisional patent application based on the work in this paper. The remaining authors declare no competing financial interests.
Figures



Comment in
-
mTOR signaling as a driver of Castleman disease.Blood. 2020 May 7;135(19):1614-1615. doi: 10.1182/blood.2020005361. Blood. 2020. PMID: 32379877 No abstract available.
References
-
- Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822-830. - PubMed
-
- Chronowski GM, Ha CS, Wilder RB, Cabanillas F, Manning J, Cox JD. Treatment of unicentric and multicentric Castleman disease and the role of radiotherapy. Cancer. 2001;92(3):670-676. - PubMed
-
- Bower M, Powles T, Williams S, et al. . Brief communication: rituximab in HIV-associated multicentric Castleman disease. Ann Intern Med. 2007;147(12):836-839. - PubMed
-
- Hoffmann C, Schmid H, Müller M, et al. . Improved outcome with rituximab in patients with HIV-associated multicentric Castleman disease. Blood. 2011;118(13):3499-3503. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

