Malignancy Rates in Brodalumab Clinical Studies for Psoriasis
- PMID: 32207067
- PMCID: PMC7275023
- DOI: 10.1007/s40257-020-00512-4
Malignancy Rates in Brodalumab Clinical Studies for Psoriasis
Abstract
Background: Brodalumab is a fully human anti-interleukin-17 receptor A monoclonal antibody efficacious for the treatment of adults with moderate-to-severe plaque psoriasis.
Objective: This study summarizes malignancy rates in psoriasis clinical studies of brodalumab.
Methods: Data were pooled from one phase II study and three large, multicenter, phase III randomized studies of brodalumab for the treatment of psoriasis, including two studies with randomization to brodalumab, ustekinumab, or placebo. Data from the 52-week (brodalumab and ustekinumab) and long-term (brodalumab) pools were summarized as exposure-adjusted or follow-up time-adjusted event rates per 100 patient-years (PY).
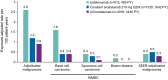
Results: Exposure-adjusted event rates per 100 PY at 52 weeks were lower with brodalumab (n = 4019; 3446 total PY of exposure) than with ustekinumab (n = 613; 495 total PY of exposure), including adjudicated malignancies (0.9 vs 2.6) and Surveillance, Epidemiology, and End Results (SEER)-adjudicated malignancies (0.3 vs 0.4). The exposure-adjusted event rate of adjudicated malignancies in the brodalumab group remained stable in the long-term analysis (0.9 [82 events]).
Conclusions: Rates of malignancy among brodalumab-treated patients with psoriasis were generally low.
Trial registry: ClinicalTrials.gov identifier NCT00975637; NCT01101100; NCT01708590 (AMAGINE-1); NCT01708603 (AMAGINE-2); NCT01708629 (AMAGINE-3).
Conflict of interest statement
Alice Gottlieb has served as a consultant or as an advisory board member for Janssen, Celgene, Bausch Health, Bristol-Myers Squibb, Beiersdorf, AbbVie, UCB, Novartis, Incyte, Eli Lilly, Dr. Reddy’s Laboratories, Dermira, Allergan, Sun Pharma, XBiotech, LEO Pharmaceuticals, Avotres Therapeutics, and Boehringer Ingelheim and has received research or educational grants from Janssen, Incyte, Novartis, XBiotech, UCB, and Boehringer Ingelheim. Mark Lebwohl is an employee of Mount Sinai, which receives research funds from AbbVie, Amgen, Arcutis, AstraZeneca, Boehringer Ingelheim, Celgene, Clinuvel, Eli Lilly, Incyte, Janssen Research & Development, LLC, Kadmon Corp., LLC, LEO Pharmaceuticals, Medimmune, Novartis, Ortho Dermatologics, Pfizer, Sciderm, UCB, Inc., and ViDac and has been a consultant for Allergan, Almirall, Arcutis, Inc., Avotres Therapeutics, BirchBioMed Inc., Boehringer Ingelheim, Bristol-Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Evelo, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharmaceuticals, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Theravance, and Verrica. Clive Liu has served on speaker bureaus and participated in research and advisory boards for AbbVie, Celgene, Novartis, Lilly, Regeneron, Sanofi, Sun Pharma, Ortho Dermatologics, and Janssen. Robert J. Israel is an employee of Bausch Health US, LLC (an affiliate of Bausch Health Companies Inc.) and holds stock and/or stock options in the company. Abby Jacobson is an employee of Ortho Dermatologics (a division of Bausch Health US, LLC) and holds stocks and/or stock options in Bausch Health.
Figures
References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical