Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2
- PMID: 32207377
- PMCID: PMC7144318
- DOI: 10.1080/22221751.2020.1743767
Establishment and validation of a pseudovirus neutralization assay for SARS-CoV-2
Abstract
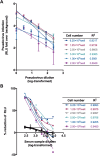
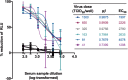
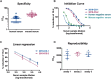
Pseudoviruses are useful virological tools because of their safety and versatility, especially for emerging and re-emerging viruses. Due to its high pathogenicity and infectivity and the lack of effective vaccines and therapeutics, live SARS-CoV-2 has to be handled under biosafety level 3 conditions, which has hindered the development of vaccines and therapeutics. Based on a VSV pseudovirus production system, a pseudovirus-based neutralization assay has been developed for evaluating neutralizing antibodies against SARS-CoV-2 in biosafety level 2 facilities. The key parameters for this assay were optimized, including cell types, cell numbers, virus inoculum. When tested against the SARS-CoV-2 pseudovirus, SARS-CoV-2 convalescent patient sera showed high neutralizing potency, which underscore its potential as therapeutics. The limit of detection for this assay was determined as 22.1 and 43.2 for human and mouse serum samples respectively using a panel of 120 negative samples. The cutoff values were set as 30 and 50 for human and mouse serum samples, respectively. This assay showed relatively low coefficient of variations with 15.9% and 16.2% for the intra- and inter-assay analyses respectively. Taken together, we established a robust pseudovirus-based neutralization assay for SARS-CoV-2 and are glad to share pseudoviruses and related protocols with the developers of vaccines or therapeutics to fight against this lethal virus.
Keywords: COVID-19; SARS-CoV-2; neutralization assay; neutralizing antibody; pseudovirus.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

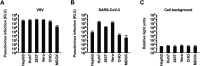
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
