Mast Cells Populate the Corneoscleral Limbus: New Insights for Our Understanding of Limbal Microenvironment
- PMID: 32207813
- PMCID: PMC7401584
- DOI: 10.1167/iovs.61.3.43
Mast Cells Populate the Corneoscleral Limbus: New Insights for Our Understanding of Limbal Microenvironment
Abstract
Purpose: Although stem cell activity represents a crucial feature in corneal and ocular surface homeostasis, other cells populating this region and the neighboring zones might participate and influence local microenvironment. Mast cells, the long-lived and tissue-sited immune cells, have been previously reported in corneoscleral specimens. Herein, mast cells were investigated in corneoscleral tissues and related to microenvironment protein expression.
Methods: Twenty-six (14 male/12 female; older than 60 years) human corneoscleral specimens were sectioned for light and fluorescent immunostaining (CD45, p63, Ck-3/7/12/19, tryptase/AA1, and chymase/CC1). Corneal, limbal, and conjunctival squares were produced for molecular and biochemical analysis. Statistical comparisons were carried out by ANOVA.
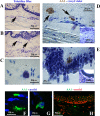
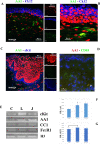
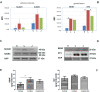
Results: Toluidine blue staining identified metachromatic intact or degranulated mast cells in the area below the palisades' Vogt (Ck-3/12-positive epithelium and underneath p63 immunoreactivity). Tryptase immunoreactivity was observed close to palisades' Vogt, whereas no specific signal was detected for chymase. Tryptase/AA1 transcripts were quantified in limbal and conjunctival RNA extracts, whereas no specific amplification was detected in corneal ones. Few mediators were overexpressed in limbal extracts with respect to corneal (Neural cell adhesion molecule (NCAM), Intercellular adhesion molecule 3 (ICAM3), Brain-derived Neurotrophic factor (BDNF), and neurotrophin 3 (NT3); P < 0.00083) and conjunctival (NCAM, ICAM3, and NT3; P < 0.05) protein extracts. A trend to an increase was observed for Nerve Growth Factor (NGF) in limbal extracts (P > 0.05).
Conclusions: The specific observation of tryptase phenotype and the interesting protein signature of microenvironment (adhesion molecules, growth factors, and neurotrophins), known to partake mast cell behavior, at least in other areas, would provide additional information to better understand this crucial zone in the framework of ocular surface healthiness.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture.Invest Ophthalmol Vis Sci. 2002 Apr;43(4):987-94. Invest Ophthalmol Vis Sci. 2002. PMID: 11923238
-
Limbal stem cells of the corneal epithelium.Surv Ophthalmol. 2000 Mar-Apr;44(5):415-25. doi: 10.1016/s0039-6257(00)00109-0. Surv Ophthalmol. 2000. PMID: 10734241 Review.
-
Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions.Mol Vis. 2007 Oct 16;13:1934-41. Mol Vis. 2007. PMID: 17982417 Free PMC article.
-
Characterization of the corneal surface in limbal stem cell deficiency and after transplantation of cultivated limbal epithelium.Ophthalmology. 2009 Jun;116(6):1048-56. doi: 10.1016/j.ophtha.2009.01.005. Epub 2009 Apr 25. Ophthalmology. 2009. PMID: 19394701
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Autophagy in Extracellular Matrix and Wound Healing Modulation in the Cornea.Biomedicines. 2022 Feb 1;10(2):339. doi: 10.3390/biomedicines10020339. Biomedicines. 2022. PMID: 35203548 Free PMC article. Review.
-
Tryptase and Exogenous Trypsin: Mechanisms and Ophthalmic Applications.J Inflamm Res. 2023 Mar 2;16:927-939. doi: 10.2147/JIR.S402900. eCollection 2023. J Inflamm Res. 2023. PMID: 36891173 Free PMC article. Review.
-
Ocular Surface Failure in Urban Syndrome.J Clin Med. 2021 Jul 9;10(14):3048. doi: 10.3390/jcm10143048. J Clin Med. 2021. PMID: 34300214 Free PMC article.
-
Unveiling the Molecular Mechanisms Underlying the Success of Simple Limbal Epithelial Transplantation (SLET).Cells. 2025 Jan 29;14(3):200. doi: 10.3390/cells14030200. Cells. 2025. PMID: 39936991 Free PMC article. Review.
-
Trackins (Trk-Targeting Drugs): A Novel Therapy for Different Diseases.Pharmaceuticals (Basel). 2024 Jul 19;17(7):961. doi: 10.3390/ph17070961. Pharmaceuticals (Basel). 2024. PMID: 39065809 Free PMC article. Review.
References
-
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997; 349: 990–993. - PubMed
-
- Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006; 24: 86–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

