Computational Design and Synthesis of a Deeply Red-Shifted and Bistable Azobenzene
- PMID: 32207943
- PMCID: PMC7307923
- DOI: 10.1021/jacs.9b10430
Computational Design and Synthesis of a Deeply Red-Shifted and Bistable Azobenzene
Abstract
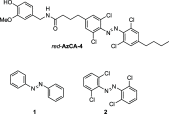
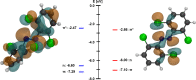
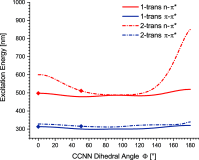
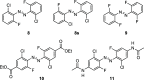
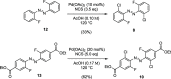
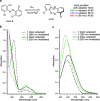
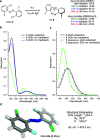
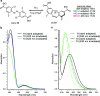
We computationally dissected the electronic and geometrical influences of ortho-chlorinated azobenzenes on their photophysical properties. X-ray analysis provided the insight that trans-tetra-ortho-chloro azobenzene is conformationally flexible and thus subject to molecular motions. This allows the photoswitch to adopt a range of red-shifted geometries, which account for the extended n → π* band tails. On the basis of our results, we designed the di-ortho-fluoro di-ortho-chloro (dfdc) azobenzene and provided computational evidence for the superiority of this substitution pattern to tetra-ortho-chloro azobenzene. Thereafter, we synthesized dfdc azobenzene by ortho-chlorination via 2-fold C-H activation and experimentally confirmed its structural and photophysical properties through UV-vis, NMR, and X-ray analyses. The advantages include near-bistable isomers and an increased separation of the n → π* bands between the trans- and cis-conformations, which allows for the generation of unusually high levels of the cis-isomer by irradiation with green/yellow light as well as red light within the biooptical window.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
A tetra-ortho-Chlorinated Azobenzene Molecule for Visible-Light Photon Energy Conversion and Storage.Molecules. 2025 May 27;30(11):2333. doi: 10.3390/molecules30112333. Molecules. 2025. PMID: 40509220 Free PMC article.
-
Red-light modulated ortho-chloro azobenzene photoswitch for peptide stapling via aromatic substitution.RSC Chem Biol. 2023 Oct 19;5(1):49-54. doi: 10.1039/d3cb00176h. eCollection 2024 Jan 3. RSC Chem Biol. 2023. PMID: 38179193 Free PMC article.
-
Synthesis of Redshifted Azobenzene Photoswitches by Late-Stage Functionalization.Chemistry. 2016 Mar 18;22(13):4364-8. doi: 10.1002/chem.201505061. Epub 2016 Feb 17. Chemistry. 2016. PMID: 26889884
-
Red-Shifting Azobenzene Photoswitches for in Vivo Use.Acc Chem Res. 2015 Oct 20;48(10):2662-70. doi: 10.1021/acs.accounts.5b00270. Epub 2015 Sep 28. Acc Chem Res. 2015. PMID: 26415024
-
Manipulation of Chemistry and Biology with Visible Light Using Tetra-ortho-Substituted Azobenzenes and Azonium Ions.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202423506. doi: 10.1002/anie.202423506. Epub 2025 Apr 10. Angew Chem Int Ed Engl. 2025. PMID: 40152740 Free PMC article. Review.
Cited by
-
Expanding excitation wavelengths for azobenzene photoswitching into the near-infrared range via endothermic triplet energy transfer.Chem Sci. 2021 Apr 27;12(21):7504-7509. doi: 10.1039/d1sc01717a. Chem Sci. 2021. PMID: 34163841 Free PMC article.
-
Theoretical evaluation of a bulky ortho-thioalkyl-azobenzene as an alternative to photocontrol structural cytotoxic effects of metal-free and disulfide oxidized hSOD1 in pathogenesis of ALS.RSC Adv. 2025 Mar 24;15(12):9018-9026. doi: 10.1039/d4ra08972c. eCollection 2025 Mar 21. RSC Adv. 2025. PMID: 40129635 Free PMC article.
-
Near-infrared light-activated Z-to-E isomerization of azobenzene via triplet sensitization from PbS quantum dots.Chem Sci. 2025 Jul 31. doi: 10.1039/d5sc03719k. Online ahead of print. Chem Sci. 2025. PMID: 40800058 Free PMC article.
-
Azobenzene/Tetraethyl Ammonium Photochromic Potassium Channel Blockers: Scope and Limitations for Design of Para-Substituted Derivatives with Specific Absorption Band Maxima and Thermal Isomerization Rate.Int J Mol Sci. 2021 Dec 6;22(23):13171. doi: 10.3390/ijms222313171. Int J Mol Sci. 2021. PMID: 34884976 Free PMC article.
-
New tricks and emerging applications from contemporary azobenzene research.Photochem Photobiol Sci. 2022 Oct;21(10):1719-1734. doi: 10.1007/s43630-022-00262-8. Epub 2022 Jul 27. Photochem Photobiol Sci. 2022. PMID: 35896915
References
-
- Hartley G. S. The Cis-form of Azobenzene. Nature 1937, 140, 281.10.1038/140281a0. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

