Phenotypic Diversity Caused by Differential Expression of SFTPC-Cre-Transgenic Alleles
- PMID: 32208105
- PMCID: PMC7258818
- DOI: 10.1165/rcmb.2019-0416MA
Phenotypic Diversity Caused by Differential Expression of SFTPC-Cre-Transgenic Alleles
Abstract
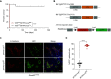
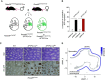
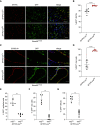
Type II alveolar epithelial cells (AEC2s) play an essential role in the function and maintenance of the pulmonary epithelium. Several transgenic mice have been developed to study the function of these cells in vivo by using the human SFTPC promoter to drive expression of Cre recombinase. The precise activity of each of these transgenic alleles has not been studied, and previous reports suggest that their activity can depend on breeding strategies. We bred mice with a conditional allele of the essential telomere capping protein TRF2 with two different SFTPC-Cre-transgenic strains and observed opposite phenotypes (100% lethality vs. 100% viability). We characterized the Cre recombinase activity in these two transgenic lines and found that the contrasting phenotypes were driven by difference in embryonic expression of the two transgenes, likely due to position effects or differences in the transgenic constructs. We also tested if SFTPC-Cre activity was dependent on maternal or paternal inheritance. When paternally inherited, both SFTPC-Cre alleles produced offspring with constitutive reporter activity independent of the inheritance of the Cre allele, suggesting that Cre recombinase was expressed in the male germline before meiosis. Immunohistochemical analysis of the testis showed reporter activity during spermatogenesis. Analysis of single-cell RNA sequencing data from murine and human testis demonstrated SFTPC expression uniquely during human spermatogenesis, suggesting that use of the human promoter in these constructs is responsible for male germline activity. Our data highlight the importance of careful analysis of transgenic allele activity and identify an SFTPC-Cre allele that is useful for panepithelial targeting in the mouse.
Keywords: SFTPC; lineage tracing; lung development.
Figures
Similar articles
-
Incomplete cre-mediated excision leads to phenotypic differences between Stra8-iCre; Mov10l1(lox/lox) and Stra8-iCre; Mov10l1(lox/Δ) mice.Genesis. 2013 Jul;51(7):481-90. doi: 10.1002/dvg.22389. Epub 2013 Mar 30. Genesis. 2013. PMID: 23554062 Free PMC article.
-
Efficiency and Specificity of Gene Deletion in Lung Epithelial Doxycycline-Inducible Cre Mice.Am J Respir Cell Mol Biol. 2017 Aug;57(2):248-257. doi: 10.1165/rcmb.2016-0208OC. Am J Respir Cell Mol Biol. 2017. PMID: 28287822 Free PMC article.
-
Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes.Mamm Genome. 2000 Mar;11(3):196-205. doi: 10.1007/s003350010037. Mamm Genome. 2000. PMID: 10723724
-
Tools and Techniques for Wt1-Based Lineage Tracing.Methods Mol Biol. 2016;1467:41-59. doi: 10.1007/978-1-4939-4023-3_4. Methods Mol Biol. 2016. PMID: 27417958 Review.
-
Conditional gene knockout system in cone photoreceptors.Adv Exp Med Biol. 2006;572:173-8. doi: 10.1007/0-387-32442-9_26. Adv Exp Med Biol. 2006. PMID: 17249572 Review.
Cited by
-
Type II alveolar epithelial cell aryl hydrocarbon receptor protects against allergic airway inflammation through controlling cell autophagy.Front Immunol. 2022 Jul 22;13:964575. doi: 10.3389/fimmu.2022.964575. eCollection 2022. Front Immunol. 2022. PMID: 35935956 Free PMC article.
-
Application of single-cell RNA sequencing on human testicular samples: a comprehensive review.Int J Biol Sci. 2023 Apr 9;19(7):2167-2197. doi: 10.7150/ijbs.82191. eCollection 2023. Int J Biol Sci. 2023. PMID: 37151874 Free PMC article. Review.
-
The CaT stretcher: An open-source system for delivering uniaxial strain to cells and tissues (CaT).Front Bioeng Biotechnol. 2022 Oct 18;10:959335. doi: 10.3389/fbioe.2022.959335. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36329705 Free PMC article.
-
Type II alveolar epithelial cell-specific loss of RhoA exacerbates allergic airway inflammation through SLC26A4.JCI Insight. 2021 Jul 22;6(14):e148147. doi: 10.1172/jci.insight.148147. JCI Insight. 2021. PMID: 34101619 Free PMC article.
-
Lung Epithelium Releases Growth Differentiation Factor 15 in Response to Pathogen-mediated Injury.Am J Respir Cell Mol Biol. 2024 May;70(5):379-391. doi: 10.1165/rcmb.2023-0429OC. Am J Respir Cell Mol Biol. 2024. PMID: 38301257 Free PMC article.
References
-
- Branda CS, Dymecki SM. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. - PubMed
-
- Sternberg N, Hoess R. The molecular genetics of bacteriophage P1. Annu Rev Genet. 1983;17:123–154. - PubMed
-
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

