Performance Characteristics of the BluePrint® Breast Cancer Diagnostic Test
- PMID: 32208353
- PMCID: PMC7097521
- DOI: 10.1016/j.tranon.2020.100756
Performance Characteristics of the BluePrint® Breast Cancer Diagnostic Test
Abstract
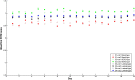
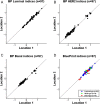
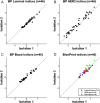
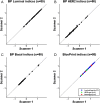
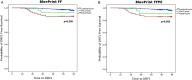
The analytical performance of a multi-gene diagnostic signature depends on many parameters, including precision, repeatability, reproducibility and intra-tumor heterogeneity. Here we study the analytical performance of the BluePrint 80-gene breast cancer molecular subtyping test through determination of these performance characteristics. BluePrint measures the expression of 80 genes that assess functional pathways which determine the intrinsic breast cancer molecular subtypes (i.e. Luminal-type, HER2-type, Basal-type). Knowing a tumor's dominant functional pathway can help allocate effective treatment to appropriate patients. Here we show that BluePrint is a highly precise and highly reproducible test with correlations above 98% based on the generated index and subtype concordance above 99%. Therefore, BluePrint can be used as a robust and reliable tool to identify breast cancer molecular subtypes.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Krijgsman O. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat. 2012;133:37–47. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

