Aberrant cell migration contributes to defective airway epithelial repair in childhood wheeze
- PMID: 32208383
- PMCID: PMC7205257
- DOI: 10.1172/jci.insight.133125
Aberrant cell migration contributes to defective airway epithelial repair in childhood wheeze
Abstract
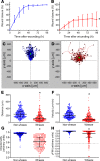
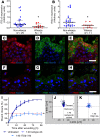
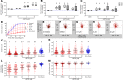
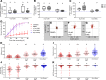
Abnormal wound repair has been observed in the airway epithelium of patients with chronic respiratory diseases, including asthma. Therapies focusing on repairing vulnerable airways, particularly in early life, present a potentially novel treatment strategy. We report defective lower airway epithelial cell repair to strongly associate with common pre-school-aged and school-aged wheezing phenotypes, characterized by aberrant migration patterns and reduced integrin α5β1 expression. Next generation sequencing identified the PI3K/Akt pathway as the top upstream transcriptional regulator of integrin α5β1, where Akt activation enhanced repair and integrin α5β1 expression in primary cultures from children with wheeze. Conversely, inhibition of PI3K/Akt signaling in primary cultures from children without wheeze reduced α5β1 expression and attenuated repair. Importantly, the FDA-approved drug celecoxib - and its non-COX2-inhibiting analogue, dimethyl-celecoxib - stimulated the PI3K/Akt-integrin α5β1 axis and restored airway epithelial repair in cells from children with wheeze. When compared with published clinical data sets, the identified transcriptomic signature was also associated with viral-induced wheeze exacerbations highlighting the clinical potential of such therapy. Collectively, these results identify airway epithelial restitution via targeting the PI3K-integrin α5β1 axis as a potentially novel therapeutic avenue for childhood wheeze and asthma. We propose that the next step in the therapeutic development process should be a proof-of-concept clinical trial, since relevant animal models to test the crucial underlying premise are unavailable.
Keywords: Asthma; Cell Biology; Cell migration/adhesion; Integrins; Pulmonology.
Conflict of interest statement
Figures



References
-
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. - PubMed
-
- O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW, START Investigators Group Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

