Histopathologic assessment of cultured human thymus
- PMID: 32208448
- PMCID: PMC7093005
- DOI: 10.1371/journal.pone.0230668
Histopathologic assessment of cultured human thymus
Abstract
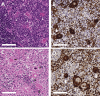
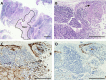
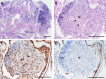
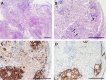
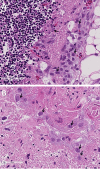
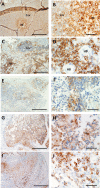
The maintenance and propagation of complex mixtures of cells in vitro in the form of native organs or engineered organoids has contributed to understanding mechanisms of cell and organ development and function which can be translated into therapeutic benefits. For example, allogeneic cultured postnatal human thymus tissue has been shown to support production of naïve recipient T cells when transplanted into patients with complete DiGeorge anomaly and other genetic defects that result in congenital lack of a thymus. Patients receiving such transplants typically exhibit reversal of their immunodeficiency and normalization of their peripheral blood T cell receptor V-beta repertoire, with long-term survival. This study was designed to assess the histopathologic changes that occur in postnatal human thymus slices when cultured according to protocols used for transplanted tissues. Results showed that as thymic organ cultures progressed from days 0 through 21, slices developed increasing amounts of necrosis, increasing condensation of thymic epithelium, and decreasing numbers of residual T cells. The architecture of the thymic epithelial network remained generally well-preserved throughout the 21 days of culture, with focal expression of cytokeratin 14, a putative biomarker of thymic epithelial cells with long-term organ-repopulating potential. All organ slices derived from the same donor thymus closely resembled one another, with minor differences in size, shape, and relative content of cortex versus medulla. Similarly, slices derived from different donors showed similar histopathologic characteristics when examined at the same culture time point. Taken together, these results demonstrate that diagnostic criteria based on structural features of the tissue identifiable via hematoxylin and eosin staining and cytokeratin immunohistochemistry can be used to evaluate the quality of slices transplanted into patients with congenital athymia.
Conflict of interest statement
Cultured postnatal human thymus (RVT-802) is an investigational product implanted into patients under an Investigational New Drug (IND) application with the United States Food and Drug Administration (FDA) that is sponsored by Dr. Markert. The technology for RVT-802 was developed by Dr. Markert and has been licensed to Enzyvant Therapeutics GmbH (Enzyvant) by Duke University. Dr. Markert and Duke University have received royalties from Enzyvant. If the technology is commercially successful in the future, Dr. Markert and Duke University may benefit financially. Portions of the salaries of Drs. Markert, Kurtzberg, and Hale and consultation fees for Dr. Neff were paid by funding to Duke University from Enzyvant. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures








References
-
- Markert ML, McLaughlin TM, Davis CM, Watson TJ, Ward FE, Kostyu D, et al. Successful formation of a chimeric human thymus allograft following transplantation of partially HLA-matched postnatal thymus. J Immunol. 1997;158: 998–1005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

