Neuroprotective role of lactate in rat neonatal hypoxia-ischemia
- PMID: 32208801
- PMCID: PMC7812521
- DOI: 10.1177/0271678X20908355
Neuroprotective role of lactate in rat neonatal hypoxia-ischemia
Abstract
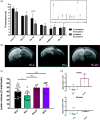
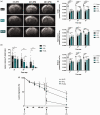
Hypoxic-ischemic (HI) encephalopathy remains a major cause of perinatal mortality and chronic disability in newborns worldwide (1-6 for 1000 births). The only current clinical treatment is hypothermia, which is efficient for less than 60% of babies. Mainly considered as a waste product in the past, lactate, in addition to glucose, is increasingly admitted as a supplementary fuel for neurons and, more recently, as a signaling molecule in the brain. Our aim was to investigate the neuroprotective effect of lactate in a neonatal (seven day old) rat model of hypoxia-ischemia. Pups received intra-peritoneal injection(s) of lactate (40 μmol). Size and apparent diffusion coefficients of brain lesions were assessed by magnetic resonance diffusion-weighted imaging. Oxiblot analyses and long-term behavioral studies were also conducted. A single lactate injection induced a 30% reduction in brain lesion volume, indicating a rapid and efficient neuroprotective effect. When oxamate, a lactate dehydrogenase inhibitor, was co-injected with lactate, the neuroprotection was completely abolished, highlighting the role of lactate metabolism in this protection. After three lactate injections (one per day), pups presented the smallest brain lesion volume and a complete recovery of neurological reflexes, sensorimotor capacities and long-term memory, demonstrating that lactate administration is a promising therapy for neonatal HI insult.
Keywords: MRI; Neonatal hypoxia-ischemia; astrocyte to neuron lactate shuttle; lactate; neuroprotection.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

