Phase-I trial of survivin inhibition with EZN-3042 in dogs with spontaneous lymphoma
- PMID: 32209084
- PMCID: PMC7092583
- DOI: 10.1186/s12917-020-02317-3
Phase-I trial of survivin inhibition with EZN-3042 in dogs with spontaneous lymphoma
Abstract
Background: Lymphoma is a common cancer in dogs. While most dogs receiving chemotherapy experience remission, very few are cured, and median survival times are generally in the 12-month range. Novel approaches to treatment are unquestionably needed. The Inhibitor of Apoptosis Protein (IAP) family member survivin, which is one of the most commonly overexpressed proteins in human cancer, plays a key role in apoptosis resistance, a major cause of drug-resistant treatment failure. Survivin targeting therapies have shown promise preclinically; however, none have been evaluated in dogs to date. The goal of the current study was to determine the safety and pharmacodynamic effects of systemic administration of the anti-survivin locked nucleic acid antisense oligonucleotide EZN-3042 in dogs with lymphoma.
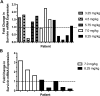
Results: We performed a prospective phase-I clinical trial in dogs with biopsy-accessible peripheral nodal lymphoma. Eighteen dogs were treated with EZN-3042 as a 2-h IV infusion at 5 dose levels, from 3.25 to 8.25 mg/kg twice weekly for 3 treatments. No dose-limiting toxicities were encountered. Reduction in tumor survivin mRNA and protein were observed in 3 of 5 evaluable dogs at the 8.25 mg/kg dose cohort.
Conclusions: In conclusion, reduced survivin expression was demonstrated in lymphoma tissues in the majority of dogs treated with EZN-3042 at 8.25 mg/kg twice weekly, which was associated with minimal adverse effects. This dose may be used in future studies of EZN-3042/chemotherapy combinations in dogs with spontaneous lymphoma and other cancers.
Keywords: Antisense; Apoptosis; Cancer; Canine; birc5.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
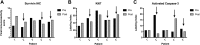
Similar articles
-
Survivin inhibition via EZN-3042 in canine lymphoma and osteosarcoma.Vet Comp Oncol. 2016 Jun;14(2):e45-57. doi: 10.1111/vco.12104. Epub 2014 Jun 13. Vet Comp Oncol. 2016. PMID: 24923332
-
Down-modulation of survivin expression and inhibition of tumor growth in vivo by EZN-3042, a locked nucleic acid antisense oligonucleotide.Nucleosides Nucleotides Nucleic Acids. 2010 Feb;29(2):97-112. doi: 10.1080/15257771003597733. Nucleosides Nucleotides Nucleic Acids. 2010. PMID: 20391197
-
A phase I study of EZN-3042, a novel survivin messenger ribonucleic acid (mRNA) antagonist, administered in combination with chemotherapy in children with relapsed acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia and lymphoma (TACL) consortium.J Pediatr Hematol Oncol. 2014 Aug;36(6):458-63. doi: 10.1097/MPH.0b013e3182a8f58f. J Pediatr Hematol Oncol. 2014. PMID: 24276047 Free PMC article. Clinical Trial.
-
Trace of survivin in cancer.Eur J Cancer Prev. 2019 Jul;28(4):365-372. doi: 10.1097/CEJ.0000000000000453. Eur J Cancer Prev. 2019. PMID: 29847456 Review.
-
Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions.Drug Resist Updat. 2002 Apr;5(2):65-72. doi: 10.1016/s1368-7646(02)00049-3. Drug Resist Updat. 2002. PMID: 12135582 Review.
Cited by
-
Expression of vascular endothelial growth factor receptor-2, epidermal growth factor receptor, cyclooxygenase-2, survivin, E-cadherin and Ki-67 in canine nasal carcinomas and sarcomas - a pilot study.Front Vet Sci. 2024 Aug 29;11:1388493. doi: 10.3389/fvets.2024.1388493. eCollection 2024. Front Vet Sci. 2024. PMID: 39268521 Free PMC article.
-
Diagnostic value of serum survivin, Ki-67 and thymidine kinase in dogs with nasal cavity disease.Front Vet Sci. 2025 Apr 7;12:1553551. doi: 10.3389/fvets.2025.1553551. eCollection 2025. Front Vet Sci. 2025. PMID: 40260215 Free PMC article.
-
Comparative Cancer Cell Signaling in Muscle-Invasive Urothelial Carcinoma of the Bladder in Dogs and Humans.Biomedicines. 2021 Oct 14;9(10):1472. doi: 10.3390/biomedicines9101472. Biomedicines. 2021. PMID: 34680588 Free PMC article. Review.
References
-
- Leukemia and Lymphoma Society. Facts, 2010-2011. White Plains: Leukemia and Lymphoma Society; 2011.
-
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29. - PubMed
-
- Vail DM, Thamm DH. Spontaneously occurring tumors in companion animals as models for drug development. In: Teicher BA, Andrews PA, editors. Anticancer Drug Development Guide: Preclinical Screening, Clinical Trials, and Approval (2nd Ed). Totowa: Humana Press; 2004. p. 259–84.
-
- Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J Vet Intern Med. 2002;16(6):704–709. - PubMed
-
- Hosoya K, Kisseberth WC, Lord LK, Alvarez FJ, Lara-Garcia A, Kosarek CE, London CA, Couto CG. Comparison of COAP and UW-19 protocols for dogs with multicentric lymphoma. J Vet Intern Med. 2007;21(6):1355–1363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

