Building customizable auto-luminescent luciferase-based reporters in plants
- PMID: 32209230
- PMCID: PMC7164954
- DOI: 10.7554/eLife.52786
Building customizable auto-luminescent luciferase-based reporters in plants
Erratum in
-
Correction: Building customizable auto-luminescent luciferase-based reporters in plants.Elife. 2020 Jul 14;9:e60938. doi: 10.7554/eLife.60938. Elife. 2020. PMID: 32662767 Free PMC article.
Abstract
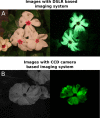
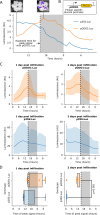
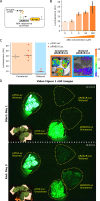
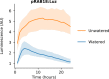
Bioluminescence is a powerful biological signal that scientists have repurposed as a reporter for gene expression in plants and animals. However, there are downsides associated with the need to provide a substrate to these reporters, including its high cost and non-uniform tissue penetration. In this work we reconstitute a fungal bioluminescence pathway (FBP) in planta using a composable toolbox of parts. We demonstrate that the FBP can create luminescence across various tissues in a broad range of plants without external substrate addition. We also show how our toolbox can be used to deploy the FBP in planta to build auto-luminescent reporters for the study of gene-expression and hormone fluxes. A low-cost imaging platform for gene expression profiling is also described. These experiments lay the groundwork for future construction of programmable auto-luminescent plant traits, such as light driven plant-pollinator interactions or light emitting plant-based sensors.
Keywords: A. thaliana; Nicotiana benthamiana; bioluminescence; expression reporter; hormone reporters; luciferase; plant biology; plant synthetic biology.
Plain language summary
Many animals have evolved the capacity to produce light from chemical reactions. For example, an enzyme known as luciferase in fireflies produces light by acting on a molecule called luciferin. Scientists have identified the enzymes that drive several of these systems and used them to build reporters that can study the activity of genes in the tissues of plants and other lifeforms over space and time. However, these reporters often require chemicals to be added to the tissues to produce light. These chemicals tend to be expensive and may not penetrate evenly into the tissues of interest, limiting the potential applications of the reporters in research studies. Recently, it has been discovered that fungi have a bioluminescence pathway that converts a molecule known as caffeic acid into luciferin. Caffeic acid is a common molecule in plants, therefore, it is possible the fungal bioluminescence pathway could be used to build reporters that produce light without needing the addition of chemicals. Now, Khakhar et al. have inserted the genes that encode the enzymes of the fungal bioluminescence pathway into tobacco plants. The experiments found that this was sufficient to turn caffeic acid into molecules of luciferin which are able to produce light. Inserting the same genes into several other plant species, including tomatoes and dahlias, produced similar results. Further experiments showed that the fungal bioluminescence pathway can be used to build reporters that monitor the activity of plant genes throughout living tissues and over a period of several days as well as examine the response to plant hormones. Alongside studying the activities of genes in plants, Khakhar et al. propose that the toolkit developed in this work could be used to generate plants with luminescence that can be switched on or off as desired. This could have many uses including helping plants attract insects to pollinate flowers and building plant biosensors that emit light in response to environmental signals.
© 2020, Khakhar et al.
Conflict of interest statement
AK, CS, JC, NL, SS, CW, RS, FR, TI, DV No competing interests declared
Figures


Comment in
-
Lighting the Way: Advances in Engineering Autoluminescent Plants.Trends Plant Sci. 2020 Dec;25(12):1176-1179. doi: 10.1016/j.tplants.2020.08.004. Epub 2020 Sep 2. Trends Plant Sci. 2020. PMID: 32891561
References
-
- Chamness JC. Programmable Plant Photography Platform. eda3ce8GitHub. 2020 https://github.com/craftyKraken/P4
-
- Davin LB, Lewis NG. Phenolic Metabolism in Plants. Boston: Springer; 1992. Phenylpropanoid metabolism: biosynthesis of monolignols, lignans and neolignans, lignins and suberins; pp. 325–375. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

