The signal peptide as a new target for drug design
- PMID: 32209293
- PMCID: PMC7138182
- DOI: 10.1016/j.bmcl.2020.127115
The signal peptide as a new target for drug design
Abstract
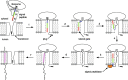
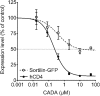
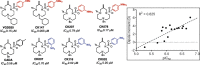
Many current and potential drug targets are membrane-bound or secreted proteins that are expressed and transported via the Sec61 secretory pathway. They are targeted to translocon channels across the membrane of the endoplasmic reticulum (ER) by signal peptides (SPs), which are temporary structures on the N-termini of their nascent chains. During translation, such proteins enter the lumen and membrane of the ER by a process known as co-translational translocation. Small molecules have been found that interfere with this process, decreasing protein expression by recognizing the unique structures of the SPs of particular proteins. The SP may thus become a validated target for designing drugs for numerous disorders, including certain hereditary diseases.
Keywords: CADA; CD4; ER; Hereditary diseases; Inhibitor; Protein; Sec61; Signal peptide; Sortilin; Translocation.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this publication.
Figures


Similar articles
-
Signal peptide-binding drug as a selective inhibitor of co-translational protein translocation.PLoS Biol. 2014 Dec 2;12(12):e1002011. doi: 10.1371/journal.pbio.1002011. eCollection 2014 Dec. PLoS Biol. 2014. PMID: 25460167 Free PMC article.
-
A Proteomic Survey Indicates Sortilin as a Secondary Substrate of the ER Translocation Inhibitor Cyclotriazadisulfonamide (CADA).Mol Cell Proteomics. 2017 Feb;16(2):157-167. doi: 10.1074/mcp.M116.061051. Epub 2016 Dec 20. Mol Cell Proteomics. 2017. PMID: 27998951 Free PMC article.
-
Preprotein signature for full susceptibility to the co-translational translocation inhibitor cyclotriazadisulfonamide.Traffic. 2020 Feb;21(2):250-264. doi: 10.1111/tra.12713. Epub 2019 Nov 25. Traffic. 2020. PMID: 31675144 Free PMC article.
-
Inhibitors of protein translocation across membranes of the secretory pathway: novel antimicrobial and anticancer agents.Cell Mol Life Sci. 2018 May;75(9):1541-1558. doi: 10.1007/s00018-017-2743-2. Epub 2018 Jan 5. Cell Mol Life Sci. 2018. PMID: 29305616 Free PMC article. Review.
-
Take Me Home, Protein Roads: Structural Insights into Signal Peptide Interactions during ER Translocation.Int J Mol Sci. 2021 Nov 1;22(21):11871. doi: 10.3390/ijms222111871. Int J Mol Sci. 2021. PMID: 34769302 Free PMC article. Review.
Cited by
-
Role of Sec61α2 Translocon in Insulin Biosynthesis.Diabetes. 2024 Dec 1;73(12):2034-2044. doi: 10.2337/db24-0115. Diabetes. 2024. PMID: 39325584
-
Endomembrane remodeling in SARS-CoV-2 infection.Cell Insight. 2022 May 17;1(3):100031. doi: 10.1016/j.cellin.2022.100031. eCollection 2022 Jun. Cell Insight. 2022. PMID: 37193051 Free PMC article. Review.
-
Reduction of Progranulin-Induced Breast Cancer Stem Cell Propagation by Sortilin-Targeting Cyclotriazadisulfonamide (CADA) Compounds.J Med Chem. 2021 Sep 9;64(17):12865-12876. doi: 10.1021/acs.jmedchem.1c00943. Epub 2021 Aug 24. J Med Chem. 2021. PMID: 34428050 Free PMC article.
-
Complete Genome and Molecular Characterization of a New Cyprinid Herpesvirus 2 (CyHV-2) SH-01 Strain Isolated from Cultured Crucian Carp.Viruses. 2022 Sep 17;14(9):2068. doi: 10.3390/v14092068. Viruses. 2022. PMID: 36146873 Free PMC article.
-
Potential role of chimeric genes in pathway-related gene co-expression modules.World J Surg Oncol. 2021 May 12;19(1):149. doi: 10.1186/s12957-021-02248-9. World J Surg Oncol. 2021. PMID: 33980272 Free PMC article.
References
-
- Patrick G.L. 5th ed. Oxford University Press; Oxford, UK: 2013. An Introduction to Medicinal Chemistry.
-
- Hopkins A.L., Groom C.R. Opinion: the druggable genome. Nat Rev Drug Discov. 2002;1:727–730. - PubMed
-
- Chen X.P., Du G.H. Target validation: a door to drug discovery. Drug Discov Ther. 2007;1:23–29. - PubMed
-
- Deweerdt S. RNA therapies explained. Nature. 2019;574:S2–S3.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials

