Computational model predicts paracrine and intracellular drivers of fibroblast phenotype after myocardial infarction
- PMID: 32209358
- PMCID: PMC7434705
- DOI: 10.1016/j.matbio.2020.03.007
Computational model predicts paracrine and intracellular drivers of fibroblast phenotype after myocardial infarction
Abstract
The fibroblast is a key mediator of wound healing in the heart and other organs, yet how it integrates multiple time-dependent paracrine signals to control extracellular matrix synthesis has been difficult to study in vivo. Here, we extended a computational model to simulate the dynamics of fibroblast signaling and fibrosis after myocardial infarction (MI) in response to time-dependent data for nine paracrine stimuli. This computational model was validated against dynamic collagen expression and collagen area fraction data from post-infarction rat hearts. The model predicted that while many features of the fibroblast phenotype at inflammatory or maturation phases of healing could be recapitulated by single static paracrine stimuli (interleukin-1 and angiotensin-II, respectively), mimicking the reparative phase required paired stimuli (e.g. TGFβ and endothelin-1). Virtual overexpression screens simulated with either static cytokine pairs or post-MI paracrine dynamic predicted phase-specific regulators of collagen expression. Several regulators increased (Smad3) or decreased (Smad7, protein kinase G) collagen expression specifically in the reparative phase. NADPH oxidase (NOX) overexpression sustained collagen expression from reparative to maturation phases, driven by TGFβ and endothelin positive feedback loops. Interleukin-1 overexpression had mixed effects, both enhancing collagen via the TGFβ positive feedback loop and suppressing collagen via NFκB and BAMBI (BMP and activin membrane-bound inhibitor) incoherent feed-forward loops. These model-based predictions reveal network mechanisms by which the dynamics of paracrine stimuli and interacting signaling pathways drive the progression of fibroblast phenotypes and fibrosis after myocardial infarction.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declarations of Competing Interest The authors have declared that no conflict of interest exists.
Figures
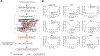
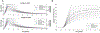
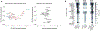



References
-
- Beltrami CA, Finato N, Rocco M, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation; 89: 151–163. - PubMed
-
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: Executive summary: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol 2013. Epub ahead of print 2013. DOI: 10.1016/j.jacc.2012.11.018. - DOI - PubMed
-
- Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: A scientific statement from the American Heart Association Councils on epidemiology and prevention, clinical cardiology, cardiovascular nursing, and high blood pressure research; Quality of Care and Outcomes Research Interdisc. Circulation 2008. Epub ahead of print 2008. DOI: 10.1161/CIRCULATIONAHA.107.188965. - DOI - PubMed
-
- Gottdiener JS, Arnold a M, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol; 35: 1628–1637. - PubMed
-
- He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med; 161: 996–1002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

